Studies on Stem Cells Research and Therapy
Bovine whey improved the myocardial and lung damage of mother rats fed on a high fat diet
Hassan IH El-Sayyad1*, Hebat A el-Ghawet1, Khaled SM El-Bayomi2 and Eman Emara1
2Anatomy Department, Faculty of Medicine, Mansoura University, Egypt
Cite this as
El-Sayyad HI, El-Ghawet HA, El-Bayomi KS, Emara E (2020) Bovine whey improved the myocardial and lung damage of mother rats fed on a high fat diet. Studies on Stem Cells Research and Therapy 6(1): 001-008. DOI: 10.17352/sscrt.000014Back ground: High fat diet associated obesity associating losing of body organs structure and function is of important public health problem. The heart and lungs represent the main organs of supplying oxygen, nutrients and nutritive molecules necessary for the body function. Little of work is concerned with higher fat diet associated myocardial and lung damage. Also, the bovine whey is rich in micronutrients with biological and medicinal activity. The present study aimed to illustrate the role of whey in ameliorating the damaged effects of higher fat diet on both heart and lung.
Methods: In the present study, one hundred and twenty fertile male and virgin female rat (Rattus norvegicus) weighing approximately 100-110g. body weight at ratio of 1 male/3 females. The experimental group fed on a high fat diet (15% fat) was carried out for 4months prior to mating. After allowing feeding on either standard or a higher fat diet for 4months, mating of virgin females were s carried out at evening and examining the sperm in vaginal sperm in the next morning and determined the onset of gestation. The pregnant categorized into four groups; control, whey supplemented group (Orally administered 1mL3), a high fat diet and a combined high fat diet and whey supplementation. At 21days-post-partum, the pregnant were sacrificed and their lung and heart were separated and fixed in 10 percent neutral buffered formalin. Histological sections stained with hematoxylin and eosin as well as immunohistochemically with p53, caspase, COX and iNOS were carried out.
Results: The present findings revealed that high fat diet induced myocardial damage with apparent fragility of the myocardial fibers, hyalinization and necrosis of myocardial fibers. Over expression of caspase 3 immunostaing and increased collagen deposition in between the myocardium was observed after masson-trichrome staining. Also, the lung tissues explained interstitial inflammatory cell infiltration with apparent fibrosis and losing alveolar spaces. The inflamed lung tissues possessed over expression of the immunostaining of COX-2, INOS and caspase 3. However, whey supplementation to the inflamed lung exhibited marked improvement and decreased expression of the assessed immunostaing.
Conclusion: Finally the authors concluded that whey contains several bioactive nutrients which facilitated reduction of the oxidative stress and supplied antioxidant components which scavenge the free radicals and ameliorated the damage associated with dietary supplementation of fat diet.
Introduction
The heart required 60%–80% of their demand of energy for pumping blood and transmission of gases and nutrients from the oxidation of fatty acids (FAs) with glucose, lactate, and ketones. Also, it used this energy in the generation of ATP [1]. Although, fat is the most calorically dense of macronutrients, the development of metabolic syndrome including obesity and type 2 diabetes is associated with intake of a higher fat diet. According to Mendoza et al. [2], dietary fat predicted obesity, elevated fasting insulin levels, and the related disorders. The incidence of obesity attained to 1.6billion overweight adults worldwide (BMI >25kg/m2) [3,4]. The WHO further predicts that, by 2015, around 2.3billion adults will be overweight and more than 700million will be obese. High fat diet associated obesity was found to alter myocardial function [5-7] and both lung stem cells and functional activity [8,9].
On the same time, bovine whey is a liquid residue of cheese, casein and yoghurt production with s a pH of 5.9-6.6. Bovine whey proteins contain important dairy ingredients rich in their amino acid content, digestibility and antioxidant activity. Consumption of whey products can modulate redox biomarkers to reduce oxidative stress. However, whey proteins themselves are targets of oxidation during processing particularly when exposed to high thermal loads. Oxidative damage of whey proteins can be associated with the degree of protein unfolding, with a-Lactalbumin more susceptible than b-Lactoglobulin [10].
Whey protein is composed of several bioactive fractions including glycomacropeptide, β-lactoglobulin, α-lactalbumin and lactoferrin, with multiple health benefits against cancer, infection and inflammation [11].
Whey protein supplementation significantly accelerated the closure of diabetic wounds by impairing inflammation and restoration the normal level of normal IL-10, TNF-α, IL-1β and IL-6 levels [12].
There is no available work illustrated the myocardial and lung damage associated intake of a higher fat diet. The present study aimed for clarification of the histopathological abnormalities and how to ameliorate by whey supplementation.
Material and methods
The study was approved by the Ethical Committee for Animal Experimentation at Faculty of Science, Mansoura University, Egypt.
Induction of a high fat diet
Experimental a high fat diet fed groups were carried out by feeding on a high fat diet composed of 15% soft animal fat mixed with stand diet contained all the standard nutritional components of protein, carbohydrate, fibers, vitamins and minerals. Virgin females were fed on a high fat diet for four months before undergoing pregnancy.
Whey syrup supplementation
Fresh bovine whey supplied from Dairy product Lab, Faculty of Agriculture, Mansoura Univ., Egypt and orally administered daily doses of 1mL3 for two months by stomach tube.
Experimental work
Forty eight virgin female rats weighing approximately 100g body weight, obtained from Hellwan Breeding Farm, Ministry of Health were used for experimentation. Free access of standard diet was supplied. Free excess of water was allowed ad-libitum. One hundred and twenty fertile male and virgin female rat (Rattus norvegicus) weighing approximately 100-110g. Body weight obtained from Hellwan Breeding Farm, Ministry of Health, Egypt and used for this work. They were housed in good ventilation with 12hour light and dark cycle. Females were mated in a special cage (1 male/3 females) during overnight and conception was determined in the next morning by the presence of sperm in a vaginal smear. The day of conception was considered to be the first day of pregnancy. The pregnant were arranged into four groups (n-20); controls, whey supplemented group, a high fat diet -group, a high fat diet & whey syrup supplementation. The pregnant of the studied groups were sacrificed after 21days post-partum. The heart and lung of mother rats were dissected and immediately, fixed in 10% phosphate-buffered formalin (pH 7.4) and processed for histological investigations. Serial 5µm thick sections were cut and stained with hematoxylin and eosin. Also, the myocardial tissue sections were stained with mason trichrome stain [13], for collagen and immunohistochemically with the antibody of p53 (3 (Thermo fisher scientific, fremont, CA, USA). In case of lung, the tissue sections were incubated with antibodies against caspase 3 (Thermo fisher scientific, Fremont, CA, USA; Cat. No. Al-70007) and stained with hematoxylin for staining of the background of tissues. The specimens were investigated with a light microscope and photographed.
Results
Myocardium
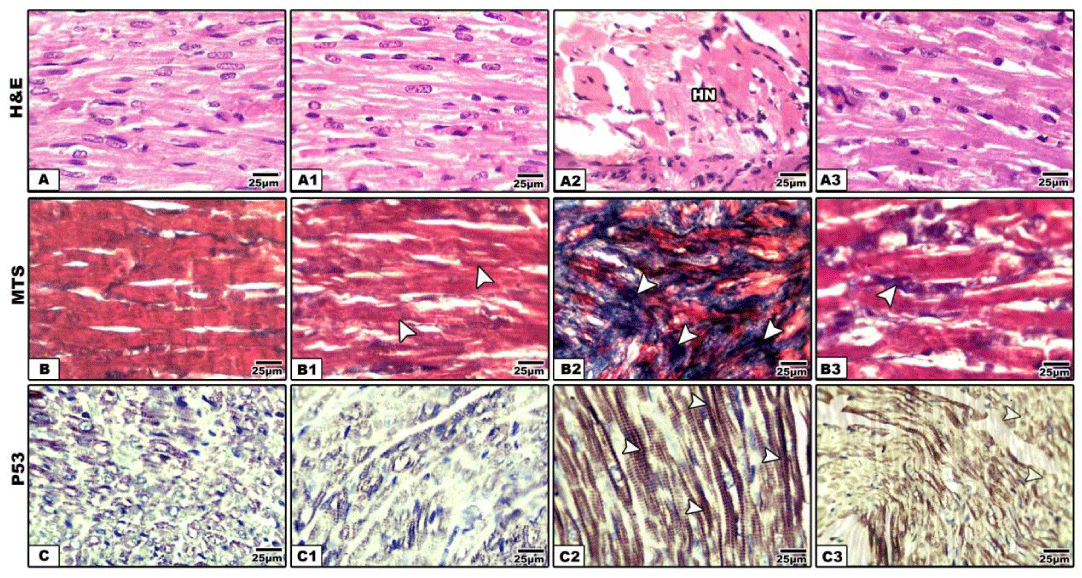
Compared with the control and whey supplementation (Figure 1A), mother rat fed on a high-fat diet exhibited eosinophilic and hyalinized necrotic muscle fibers.
Other specimens of Other specimens of myocardial tissues displayed round cell infiltration between muscle fibers (Figure 1A2). Following Masson trichrome stain, there was a detected dense collagen deposition in between the myocardial muscle fibers manifesting fibrotic change (Figure 1B2). Also, the myocardium of mother fed on a high fat diet exhibited overexpression of p53 manifesting decreased myocardial function (Figure 1C2).
Lung
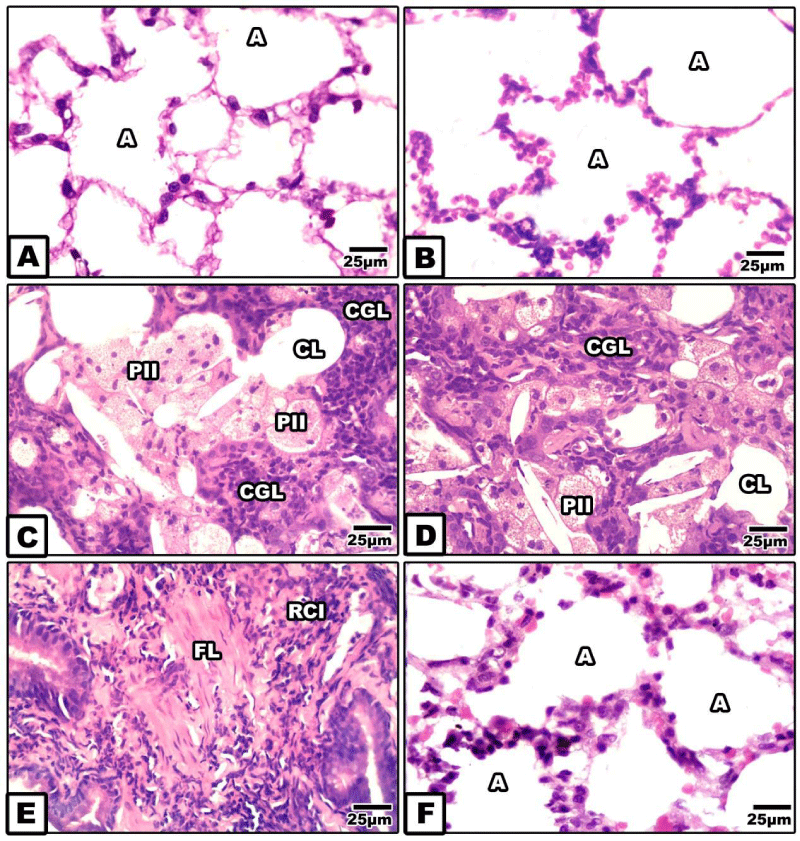
In both control and whey supplemented mother, the lung is composed of alveoli of different sizes that acquire spongy-shaped architecture.
Two groups of pneumocytes, type I & II, lined the alveolar wall which become thin and straight.
Type I cells are squamous and cover the basal lamina and the alveolar lining surface. (Figure 3A,B).
In mother fed on a high fat diet, there was a detected dense inflammation of the lung. The inflammatory cells were densely aggregated through the peri-and inner of the alveolar space losing their luminal space. In some specimens, the lung tissues possessed wide spots of interstitial fibrosis associated with hyperplasia of type II pneumocyte. Many of the type II cells become foamy, eosinophil and infiltrated throughout the alveoli. Also, fibrosis of the alveoli associated with dense collagenous deposition and surrounded by dense distributions of inflammatory cells were also detected (Figure 3C-E). On the other hand whey supplementation to mother fed on a high fat diet improved the histological picture of the alveoli (Figure 3F).
Immunohistochemistry of cysteine-aspartic acid protease 3 (caspase-3), cyclooxygenase-2COX-2) and induced nitric oxide synthase (INOS)
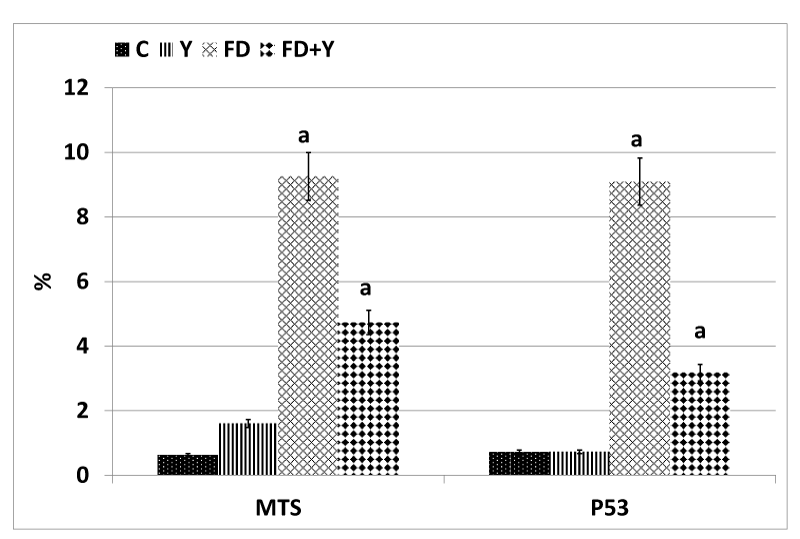
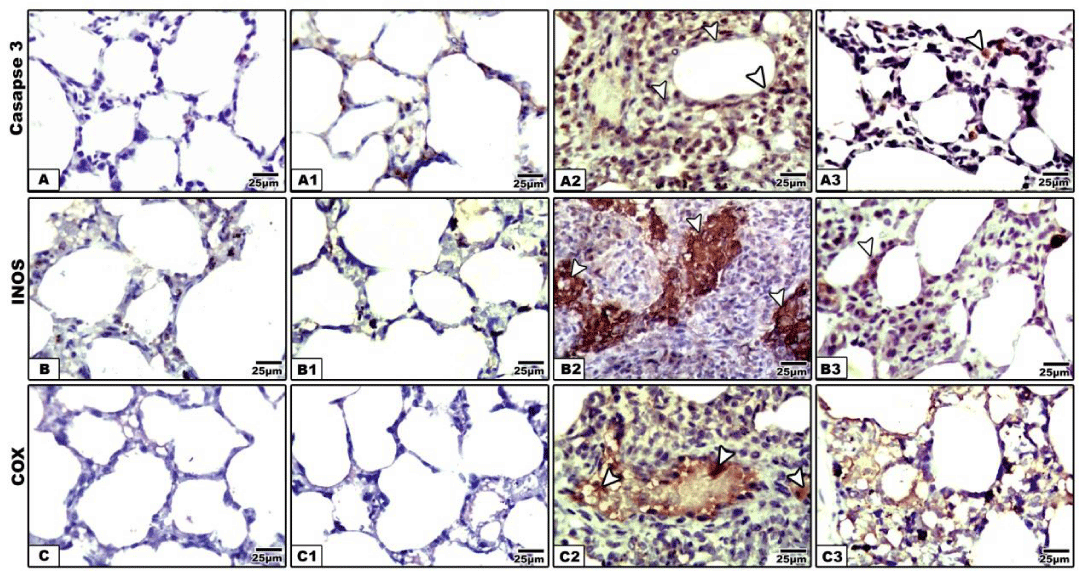
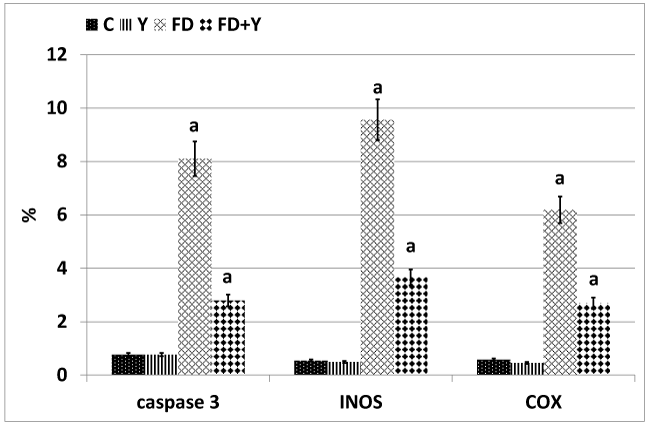
Concerning cysteine-aspartic acid protease 3 (caspase-3), the activity of caspase-3 was highly detected in the alveolar tissues of mother rats fed on a high fat diet (Figure 4A2). Mother rats fed on a high fat diet and whey supplementation possessed a moderate amelioration of the apoptic immune marker caspase-3 (Figure 4A3). Regard mild caspase 3 immunohistochemical reaction in the control and whey supplemented mothers (Figure 4A,A1). Image analysis revealed the increased intensity of the caspase-3 in a high fat diet fed mothers compared to mother fed on a high fat diet and whey supplementation (Figure 5).
In control and whey supplemented mother rats, the lung tissue possessed mild immunohistochemical reaction of COX (Figures 4B,B1). In experimental mother fed on a high fat diet, there was a marked increase of dark brown-deposits in the interstitial and alveolar wall (Figure 4B2). On the other hand whey supplementation to mother fed on a high fat diet showed a decreased immunohistochemical reaction (Figure 4B3). Image analysis revealed a marked increase of the immunostaining reaction in a high fat diet mother compared to the other studied groups (Figure 5).
Concerning inducible nitric oxide synthase immunohistochemistry, compared to the control and whey supplemented mother rats (Figure 4C,C1), the lung tissue of mother fed on a high fat diet have a comparative increase of dark brown-deposits in the interstitial tissue of the alveoli (Figure 4C2. Experimentally mother fed on a high fat diet and received whey supplementation, possessed a decreased and improved immunohistochemical reaction (Figure 4C3). Image analysis revealed a marked increase of the immunostaining reaction in a high fat diet mother compared to the other studied groups (Figure 5).
Discussion
Heart
From the present work, mother rats fed on a higher fat diet for four month as well as throughout pregnancy and lactation period exhibited fragility of the myocardial muscle fibers, hyalinization and focal collection of inflammatory cells in the necrotic zones. Masson trichrome stain possessed dense collagenous formation in between muscle fibers predicting myocardial fibrosis.
These findings were consistent with who reported myocardial hypertrophy and inflammation of mice [14], rats [15] and pigs atrophied [16] fed on a high fat diet.
The induced myocardial damage was attributed to the increased lipid burden in the myocardial tissues [17] and sarcolemma [18], which enhanced cell death and myocardial fibrosis [19, 20]. These complications influenced on left ventricular wall thickness, hypertrophy [21] and fibrosis [22].
The dramatic effects of long term ingestion of fat diet on myocardial muscle may be attributed to the fatty acid oxidation and liberation of free radicals [23], or the formation of Fatty acyl-coal from the triglyceride which increased lipid accumulation and exerted lipotoxicity.
Myocardial damage reflected pulmonary fibrosis. The inflamed lung tissues facilitated accumulation of extracellular matrix components, particularly collagen, at the site of injury [24]. At the same time acute and chronic lung inflammation is influenced in the development of cardiovascular disease [25].
The observed myocardial damage was reflected by increased p53 immunostaining.
Similar findings were observed in patients with acute myocardial infarction [26].
Beside the mentioned, mother rat fed on a high fat diet enhanced the aggregation of inflammatory cells leading to the development of fibrosis through the peri-and inner of the alveoli losing their luminal space. Interstitial fibrosis characterized by dense collagen fibers and increased the average of type II pneumocyte within lung tissues having foamy and eosinophilic structure. The death of the alveolar epithelium explained by increased caspase 3 immunohistochemically.
Similar findings of damage [27] and fibrotic lung. Were reported in obese mice fed on a high fat diet ApoE(-/-) mice fed on a high fat diet developed inflammation of the lung associated with marked deposition of collagen and increased metalloproteinase-9 matrix [30].
The damage of alveolar epithelium may result from the liberation of reactive oxygen species leading to oxidative damage to mitochondrial DNA which consequently induces cell death [31] and reflected the dietary administration of a high fat diet associated obesity [32].
The inflamed lung tissues led to fibroblast accumulation and damage of the alveolar epithelium associated the progress of idiopathic pulmonary fibrosis [33].
The observed increased inflammatory lesions were assessed by increased immunohistochemistry of cyclooxygenase-2COX-2) and induced nitric oxide synthase.
In mother rats maternal fed on a high fat diet, marked increase of COX-2 immunohistochemistry was detected within the cytoplasm of macrophages, alveolar epithelial cells, type-2 pneumocytes, and endothelial cells of blood vessels.
Similar findings of highly expressed cyclooxygenase-2 (COX-2) in AT [34].
The cyclooxygenase (COX) enzymes catalyze a key step in the conversion of arachidonate to PGH2, the immediate substrate for a series of cell specific prostaglandin and thromboxane synthases.
COX catalyzes the cyclooxygenation reaction through which arachidonic acid is enzymatically cleavage and generates the free radicals.
Inflammation of adipose tissue (AT) plays a major role in the development of many type 2 diabetes associated with obesity [35]. This inflammation is mediated by a large number of cytokines and chemokines, including TNF-α [36], IL-6 [37].
There is a direct correlation between consumption of a high fat diet and inflamed lung associated overexpression of immunostaining of cyclooxygenase 2.
The adipocyte tissues were found to enhanced expression of COX-2 inducing lung disease [38].
A high-fat diet (HFD) is known to shorten lifespan and to increase incidences of several metabolic diseases, including type-2 diabetes and various cardiovascular diseases [39].
The present findings agree with the work of Hegab et al. [8], Who reported increased number of lymphocytes and macrophages in the lung parenchyma.
Although a high fat diet does not have a direct correlation with lung cancer, the increased consumption of high fat diet increased the number of pulmonary metastases by 60% and tumor volume by 130% [40].
The overexpression of COX-2 is predicted of early diagnosed lung cancer associated with a high fat diet. The present findings supported the work of Zhu et al. [41].
Also, the observed lung inflammation of mother fed on a high fat diet exhibited increased immunohistochemical reaction of inducible nitric oxide synthase (iNOS). Inducible nitric oxide synthase (iNOS) was markedly upregulated and enhanced pulmonary disease. The overexpression of inducible nitric oxide synthase was associated with increased nitric oxide, the potent free radicle involved in damaging the alveolar epithelium and enhanced lung inflammation and fibrosis [42-44].
From the present findings, there was a detected increased collection of inflammatory cells in associated cell damage facilitated the release of free radicals associated overexpression of induced nitric oxide synthase especially in the interstitial of the alveoli.
The present findings agree with the work of whom reported increased expression of iNOS in aorta.
The present results are in consistent with the work of [45], who reported that the associated higher fat diet associated with obesity triggered macrophage increased iNOS gene expression [46]. This resulted from inducing inflammation associated type 2 diabetes [47].
From the observed findings, there were a detected improvement of fat associated the myocardium and lung tissue as well reduction of the immunostaining of p53 in heart and casp3, COX and iNOS in lung.
From the observed findings, there were a detected improvement of fat associated the myocardium and lung tissue as well reduction of the immunostaining of p53 in heart and casp3, COX and iNOS in lung.
These findings may be associated to increased whey content of sulfhydryl groups which enhanced glutathione production and reduced the in vitro production of interleukin (IL)-8 and consequently reduce the oxidative stress in cystic fibrosis [48] . The whey contents of bioactive molecules lysozyme, lactoferrin, immunoglobulins, growth factors and hormones [49], glycomacropeptide, β-lactoglobulin, α-lactalbumin and lactoferrin, with multiple health benefits against cancer, infection and inflammation [50]. These bio-components decreased creatinine kinase-MB, lactate dehydrogenase, homocysteine, triglycerides, low density lipoprotein and dyslipidemia [51], leading to myocardial and lung diseases. Supplementation of non-hydrolyzed milk protein (25g/day) for 8weeks improved vascular and endothelial function and hyperlipidemia associated diseases [52].
The authors finally concluded that whey contains bio-nutrients having antioxidant activity with therapeutic medicinal important in alleviating the dramatic changes of a higher fat diet in myocardial and lung tissues.
- Sudres M, Norol F, Trenado A, Gregoire S, Charlotte F, et al. (2006) Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol 176: 7761-7767. Link: https://bit.ly/2xc1j9L
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, et al. (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30: 42-48. Link: https://bit.ly/2xc1emt
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, et al. (2003) Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 102: 3837-3844. Link: https://bit.ly/3aTs95c
- Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, et al. (2004) Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant 33: 597-604. Link: https://go.nature.com/2Xinoy1
- Yanez R, Lamana ML, Castro JG, Colmenero I, Ramírez M, et al. (2006) Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 24: 2582-2591. Link: https://bit.ly/2RmcnYO
- Dabrowski FA, Burdzinska A, Kulesza A, Chlebus M, Kaleta B, et al. (2017) Mesenchymal Stem Cells from Human Amniotic Membrane and Umbilical Cord Can Diminish Immunological Response in an in vitro Allograft Model. Gynecol Obstet Invest 82: 267-275. Link: https://bit.ly/2RkLzId
- Nasef A, Mathieu N, Chapel A, Frick J, François S, et al. (2007) Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation 84: 231-237. Link: https://bit.ly/2URP23h
- Kim SH, Jung J, Cho KJ, Choi JH, Lee Hs, et al. (2018) Immunomodulatory Effects of Placenta-derived Mesenchymal Stem Cells on T Cells by Regulation of FoxP3 Expression. Int J Stem Cells 11: 196-204. Link: https://bit.ly/3b4rxtH
- He H, Nagamura-Inoue T, Takahashi A, Mori Y, Yamamoto Y, et al. (2015) Immunosuppressive properties of Wharton's jelly-derived mesenchymal stromal cells in vitro. Int J Hematol 102: 368-378. Link: https://bit.ly/3c1v1wQ
- Stubbendorff M, Deuse T, Hua X, Phan TT, Bieback K, et al. (2013) Immunological properties of extraembryonic human mesenchymal stromal cells derived from gestational tissue. Stem Cells Dev 22: 2619-2629. Link: https://bit.ly/2RfNkGP
- Roelen DL, van der Mast BJ, in't Anker PS, Kleijburg C, Eikmans M, et al. (2009) Differential immunomodulatory effects of fetal versus maternal multipotent stromal cells. Hum Immunol 70: 16-23. Link: https://bit.ly/2UVcPQ1
- Ding G, Wei L, Sun W, Zhang L (2015) The immunological characteristics of tonsil mesenchymal stem cells. Zhonghua Zheng Xing Wai Ke Za Zhi 31: 43-48. Link: https://bit.ly/3c1TvX2
- Vereb Z, Póliska S, Albert R, Kristoffer Olstad O, Boratkó A, et al. (2016) Role of Human Corneal Stroma-Derived Mesenchymal-Like Stem Cells in Corneal Immunity and Wound Healing. Sci Rep 6: 26227. Link: https://bit.ly/2K0GqRT
- de Mare-Bredemeijer EL, Mancham S, Verstegen MM, de Ruiter PE, van Gent R, et al. (2015) Human graft-derived mesenchymal stromal cells potently suppress alloreactive T-cell responses. Stem Cells Dev 24: 1436-1447. Link: https://bit.ly/2UQZUyd
- Li X, Bai J, Ji X, Li R, Xuan Y, et al. (2014) Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med 34: 695-704. Link: https://bit.ly/2Vgo4Bg
- Ock SA, Baregundi Subbarao R, Lee YM, Lee JH, Jeon RH, et al. (2016) Comparison of Immunomodulation Properties of Porcine Mesenchymal Stromal/Stem Cells Derived from the Bone Marrow, Adipose Tissue, and Dermal Skin Tissue. Stem Cells Int 9581350. Link: https://bit.ly/3bZ9lBr
- Talwadekar MD, Kale VP, Limaye LS (2015) Placenta-derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord-derived counterparts-a paired sample study. Sci Rep 5: 15784. Link: https://bit.ly/34l1ZWp
- Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, et al. (2006) Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 108: 2114-21120. Link: https://bit.ly/3c1U1UY
- Blanco B, Herrero-Sánchez MD, Rodríguez-Serrano C, García-Martínez ML, Blanco JF, et al. (2016) Immunomodulatory effects of bone marrow versus adipose tissue-derived mesenchymal stromal cells on NK cells: implications in the transplantation setting. Eur J Haematol 97: 528-537. Link: https://bit.ly/2VfAZDy
- Du Rocher B, Mencalha AL, Gomes BE, Abdelhay E (2012) Mesenchymal stromal cells impair the differentiation of CD14(++) CD16(-) CD64(+) classical monocytes into CD14(++) CD16(+) CD64(++) activate monocytes. Cytotherapy 14: 12-25. Link: https://bit.ly/2xWxM3R
- Bacigalupo A, Valle M, Podestà M, Pitto A, Zocchi E, et al. (2005) T-cell suppression mediated by mesenchymal stem cells is deficient in patients with severe aplastic anemia. Exp Hematol 33: 819-827. Link: https://bit.ly/2UQcRIQ
- Wang D, Feng X, Lu L, Konkel JE, Zhang H, et al. (2014) A CD8 T cell/indoleamine 2,3-dioxygenase axis is required for mesenchymal stem cell suppression of human systemic lupus erythematosus. Arthritis Rheumatol 66: 2234-2245. Link: https://bit.ly/2V97fbD
- Liu LH, Chen H, Chen B, Sun Z, Ye LP, et al. (2008) Immuno-suppressive effects on T cells mediated by mesenchymal stem cells from patients with myelodysplastic syndrome. Zhongguo Shi Yan Xue Ye Xue Za Zhi 16: 299-304. Link: https://bit.ly/34oIOLA
- Kuçi Z, Kuçi S, Zircher S, Koller S, Schubert R, et al. (2011) Mesenchymal stromal cells derived from CD271(+) bone marrow mononuclear cells exert potent allosuppressive properties. Cytotherapy 13: 1193-1204. Link: https://bit.ly/2wkyoQi
- Nasef A, Zhang YZ, Mazurier C, Bouchet S, Bensidhoum M, et al. (2009) Selected Stro-1-enriched bone marrow stromal cells display a major suppressive effect on lymphocyte proliferation. Int J Lab Hematol 31: 9-19. Link: https://bit.ly/2XicCb2
- Rasmusson I, Ringdén O, Sundberg B, Le Blanc K (2005) Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res 305: 33-41. Link: https://bit.ly/2xezyNP
- Groh ME, Maitra B, Szekely E, Koç ON (2005) Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells Exp Hematol 33: 928-934. Link: https://bit.ly/34iOPcF
- Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, et al. (2004) Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 103: 4619-4621. Link: https://bit.ly/2xaFp6E
- Nasef A, Ashammakhi N, Fouillard L (2008) Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med 3: 531-546. Link: https://bit.ly/3c5l2H3
- Luz-Crawford P1 Noël D, Fernandez X, Khoury M, Figueroa F, et al. (2012) Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS 7: e45272. Link: https://bit.ly/39SX7Jk
- Nasef A, Mazurier C, Bouchet S, François S, Chapel A, et al. (2008) Leukemia inhibitory factor: Role in human mesenchymal stem cells mediated immunosuppression. Cell Immunol 253: 16-22. Link: https://bit.ly/3aTdd75
- Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, et al. (2007) Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr 13: 217-226. Link: https://bit.ly/3aTdeHX
- Williams LB, Tessier L, Koenig JB, Koch TG (2014) Post-thaw non-cultured and post-thaw cultured equine cord blood mesenchymal stromal cells equally suppress lymphocyte proliferation in vitro. PLoS One 9: e113615. Link: https://bit.ly/3e4vIaF
- Pollock K, Sumstad D, Kadidlo D, McKenna DH, Hubel A (2015) Clinical mesenchymal stromal cell products undergo functional changes in response to freezing. Cytotherapy 17: 38-45. Link: https://bit.ly/2Xl3K4j
- Naaldijk Y, Staude M, Fedorova V, Stolzing A (2012) Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide. BMC Biotechnol 12: 49. Link: https://bit.ly/3c6TuBb
- Luetzkendorf J, Nerger K, Hering J, Moegel A, Hoffmann K, et al. (2015) Cryopreservation does not alter main characteristics of Good Manufacturing Process-grade human multipotent mesenchymal stromal cells including immunomodulating potential and lack of malignant transformation. Cytotherapy 17: 186-198. Link: https://bit.ly/2xcEUsW
- Francois M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, et al. (2012) Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy 14: 147-152. Link: https://bit.ly/2JQ6kaQ
- Chinnadurai R, Copland IB, Garcia MA, Petersen CT, Lewis CN, et al. (2016) Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNgamma Licensing. Stem Cells 34: 2429-2442. Link: https://bit.ly/3c1Vr1K
- Hoogduijn MJ, de Witte SF, Luk F, van den Hout-van Vroonhoven MC, Ignatowicz L, et al. (2016) Effects of Freeze-Thawing and Intravenous Infusion on Mesenchymal Stromal Cell Gene Expression. Stem Cells Dev 25: 586-597. Link: https://bit.ly/2wmnuJR
- Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, et al. (2014) Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 32: 2430-2442. Link: https://bit.ly/2XeqX8u
- Zhou Y, Day A, Haykal S, Keating A, Waddell TK (2013) Mesenchymal stromal cells augment CD4+ and CD8+ T-cell proliferation through a CCL2 pathway. Cytotherapy 15: 1195-1207. Link: https://bit.ly/3aSzpOP
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P (2003) Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol 171: 3426-3434. Link: https://bit.ly/2Vax0s2
- Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, et al. (2011) The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood 117: 4826-4835. Link: https://bit.ly/34jP72R
- Chen JF, Gao J, Zhang D, Wang ZH, Zhu JY, et al. (2010) CD4+Foxp3+ regulatory T cells converted by rapamycin from peripheral CD4+CD25(-) naive T cells display more potent regulatory ability in vitro. Chin Med J 123: 942-948. Link: https://bit.ly/2JQLbxb
- Sadeghi L, Kamali-Sarvestani E, Azarpira N, Shariati M, Karimi MH (2014) Immunomodulatory effects of mice mesenchymal stem cells on maturation and activation of dendritic cells. Iran J Immunol 11: 177-188. Link: https://bit.ly/3aWawBy
- Cho KA, Lee JK, Kim YH, Park M, Woo SY, et al. (2017) Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell Mol Immunol. Link: https://bit.ly/3c3BCqu
- Shin TH, Lee BC, Choi SW, Shin JH, Kang I, et al. (2017) Human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis via regulation of B lymphocyte maturation. Oncotarget 8: 512-522. Link: https://bit.ly/2UR21SF
- Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Alwayn IP, et al. (2009) Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation 87: 896-906. Link: https://bit.ly/3bXPt1N
- Kwon MS, Noh MY, Oh KW, Cho KA, Kang BY, et al. (2014) The immunomodulatory effects of human mesenchymal stem cells on peripheral blood mononuclear cells in ALS patients. J Neurochem 131: 206-218. Link: https://bit.ly/34kQWwl
- Engela AU, Baan CC, Litjens NH, Franquesa M, Betjes MG, et al. (2013) Mesenchymal stem cells control alloreactive CD8(+) CD28(-) T cells. Clin Exp Immunol 174: 449-458. Link: https://bit.ly/39Pfjn3
- Yang SH, Park MJ, Yoon IH, Kim SY, Hong SH, et al. (2009) Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp Mol Med 41: 315-324. Link: https://bit.ly/2xc82R3
- Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, et al. (2007) Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25: 2025-2032. Link: https://bit.ly/3aRdoja
- Pianta S, Bonassi Signoroni P, Muradore I, Rodrigues MF, Rossi D, et al. (2015) Amniotic membrane mesenchymal cells-derived factors skew T cell polarization toward Treg and downregulate Th1 and Th17 cells subsets. Stem Cell Rev 11: 394-407. Link: https://bit.ly/2V6oj1S
- Cahill EF, Tobin LM, Carty F, Mahon BP, English K (2015) Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther 6: 19. Link: https://bit.ly/2JPmBNi
- Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A (2007) Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 92: 881-888. Link: https://bit.ly/2Xq7L7A
- Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, et al. (2005) Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 90: 516-525. Link: https://bit.ly/2yJbG5m
- Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815-1822. Link: https://bit.ly/3aUEZQr
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, et al. (2005) Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 105: 2214-2219. Link: https://bit.ly/34iQSNT
- English K, Barry FP, Mahon BP (2008) Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett 115: 50-58. Link: https://bit.ly/2y03EV8
- Pourgholaminejad A, Aghdami N, Baharvand H, Moazzeni SM (2016) The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine 85: 51-60. Link: https://bit.ly/2wndyjk
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, et al. (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838-3843. Link: https://bit.ly/2JPO5lO
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, et al. (2003) Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101: 3722-37229. Link: https://bit.ly/39RzlNT
- Montespan F, Deschaseaux F, Sensébé L, Carosella ED, Rouas-Freiss N (2014) Osteodifferentiated mesenchymal stem cells from bone marrow and adipose tissue express HLA-G and display immunomodulatory properties in HLA-mismatched settings: implications in bone repair therapy. J Immunol Res 2014: 230346. Link: https://bit.ly/34kS96R
- Fasslrinner F, Wobus M, Duryagina R, Müller K, Stopp S, et al. (2012) Differential effects of mixed lymphocyte reaction supernatant on human mesenchymal stromal cells. Exp Hematol 40: 934-944. Link: https://bit.ly/2y2joa8
- Hong J, Hueckelhoven A, Wang L, Schmitt A, Wuchter P, et al. (2016) Indoleamine 2,3-dioxygenase mediates inhibition of virus-specific CD8(+) T cell proliferation by human mesenchymal stromal cells. Cytotherapy 18: 621-629. Link: https://bit.ly/2Rm2LNy
- Ciccocioppo R, Cangemi GC, Kruzliak P, Gallia A, Betti E, et al. (2015) Ex vivo immunosuppressive effects of mesenchymal stem cells on Crohn's disease mucosal T cells are largely dependent on indoleamine 2,3-dioxygenase activity and cell-cell contact. Stem Cell Res Ther 6: 137. Link: https://bit.ly/2JLAID6
- Wang JP, Ou-Yang GF (2014) Mechanism of HLA-G participation in inhibiting lymphocyte proliferation by amniotic mesenchymal stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 22: 187-191. Link: https://bit.ly/3e466dQ
- Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, et al. (2008) Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 26: 212-222. Link: https://bit.ly/2RmEgjj
- Najar M, Raicevic G, Boufker HI, Fayyad-Kazan H, De Bruyn C, et al. (2010) Adipose-tissue-derived and Wharton's jelly-derived mesenchymal stromal cells suppress lymphocyte responses by secreting leukemia inhibitory factor. Tissue Eng Part A 16: 3537-3346. Link: https://bit.ly/3cczVHJ
- van den Berk LC, Jansen BJ, Snowden S, Siebers-Vermeulen KG, Gilissen C, et al. (2014) Cord blood mesenchymal stem cells suppress DC-T Cell proliferation via prostaglandin B2. Stem Cells Dev 23: 1582-1593. Link: https://bit.ly/2VgrRhY
- Bocelli-Tyndall C, Bracci L, Spagnoli G, Braccini A, Bouchenaki M, et al. (2007) Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and auto-immune disease patients reduce the proliferation of autologous- and allogeneic-stimulated lymphocytes in vitro. Rheumatolog 46: 403-408. https://bit.ly/3c2q3QE
- Fekete N, Erle A, Amann EM, Fürst D, Rojewski MT, et al. (2015) Effect of high-dose irradiation on human bone-marrow-derived mesenchymal stromal cells. Tissue Eng Part C Methods 21: 112-122. Link: https://bit.ly/39LVeOA
- Wu LW, Wang YL, Christensen JM, Khalifian S, Schneeberger S, et al. (2014) Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl Immunol 30: 122-127. https://bit.ly/2UR0pZ9
- Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, et al. (2016) International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 18: 151-159. https://bit.ly/3e0YZDf
- Jenhani F (2013) Expression Profile of Galectins (Gal-1, Gal-9, Gal-11 and Gal-13) in Human Bone Marrow Derived Mesenchymal Stem Cells in Different Culture Mediums. Stem Cell Biology in Normal Life and Diseases, Kamran Alimoghaddam, Intech Open. Link: https://bit.ly/2JLLM2X
- Sierra Parraga JM, Rozenberg K, Eijken M, Leuvenink HG, Hunter J, et al. (2019) Effects of Normothermic Machine Perfusion Conditions on Mesenchymal Stromal Cells. Front Immunol 10: 765. Link: https://bit.ly/39U3Q5Q
- Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L, et al. (2013) Immunological characterization of multipotent mesenchymal stromal cells–The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 15: 1054-1061. Link: https://bit.ly/3c2qsTa
- Chinnadurai R, Rajan D, Qayed M, Arafat D, Garcia M, et al. (2018) Potency Analysis of Mesenchymal Stromal Cells Using a Combinatorial Assay Matrix Approach. Cell Reports 2504-2517. Link: https://bit.ly/34l6sbD
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
