Studies on Stem Cells Research and Therapy
Evaluation of success and toxicity of autologous stem cell transplantation in patients with multiple myeloma in the geriatric age group
Mehmet Ali Uçar1*, Simten Dağdaş2, Funda Ceran2, Mesude Falay2 and Gülsüm Özet2
2Ankara Numune Training and Research Hospital, Turkey
Cite this as
Uçar MA, Dağdaş S, Ceran F, Falay M, Özet G (2019) Evaluation of success and toxicity of autologous stem cell transplantation in patients with multiple myeloma in the geriatric age group. Studies on Stem Cells Research and Therapy 5(1): 001-006. DOI: 10.17352/sscrt.000012Background: Our objective in this study is to compare the outcomes of patients with multiple myeloma (MM) over 65 years of age who had autologous hematopoietic stem cell transplantation (AHSCT) with the outcomes of patients under 65 years of age.
Method: The study population comprised 29 patients over 65 years of age and 39 patients under 65 years of age. Records on diagnosis, stage, and treatment before and after transplantation of the patients included in the study were collected. Outcomes of patients’ initial transplantations were evaluated.
Results: The rate of positive and complex cytogenetic anomalies in patients over and under 65 years of age was determined to be 24.1% versus 5.1% (p=0.036) and 3.4% versus 2.6% (p=0.017), respectively. All of the patients who were determined to have 17p deletion, 13q deletion, and T (4;14) cytogenetic anomaly were in the older age group. In addition to this, the ratio of the patients who develop mucositis after AHSCT (p<0.001) and the ratio of the patients who developed neutropenic fever (p=0.027) were determined to be higher in the older age population. However, the rate of 1st year response to AHSCT was higher in patients over 65 years of age. Additionally, mean survival time and 5-year survival rate were significantly higher in the younger patient group (log rank p=0.020) (p<0.05).
Conclusion: In the treatment of MM, advanced age is considered to be a problem not only for AHSCT but also for intensive chemotherapies.
Introduction
Multiple myeloma (MM) is a clonal stem cell disease that occurs as a result of malignant transformation of plasma cells. It accounts for 1% of all cancers and 10% of hematological malignancies. It is more common in males compared to females (M/F: 1.4/1). Mean age at the time of diagnosis is 66; the ratio of patients under 55 years of age is less than 5%. Osteopenia, lytic lesions of bones, anemia, renal insufficiency, hypercalcemia, and infections are among the common possible complications. Complete cure cannot be achieved with the current treatments used in cases of MM [1].
The superiority of autologous hematopoietic stem cell transplantation (AHSCT) to conventional chemotherapies for treatment of patients of appropriate age who are diagnosed with MM has been proven in randomized controlled studies [2,3]. Recently, AHSCT with 200 mg/m2 of melphalan following an induction chemotherapy combined with new agents is being used as the standard treatment for patients with MM over 65 years of age [4,5]. Some of the studies evaluating the association of AHSCT applied to patients with MM with age suggest that AHSCT has a high risk of melphalan-associated toxicity in patients with MM over 65 years of age. In contrast, there are some studies proving the benefit and safety of AHSCT in the treatment of these patients [6,7]. The general view is that the risk of toxicity may be reduced in the treatment of such older patients by lowering the dose of melphalan [8].
In recent studies, it has been observed that life analyses performed on patients with MM are more superior compared to the past, and this advantage is thought to be provided by the mechanism of action of new agents. Although AHSCT is an intensive and costly procedure, it reduces the need for treatment, time to remission, and other economic problems caused by new treatments. Furthermore, it is a quite effective treatment option that may also be used as a salvage treatment in relapse-refractory disease [9,10].
Various factors such as stage of disease, presence of poor prognostic markers, accompanying comorbidities, and age play an important role in the success of transplantation in patients with MM [11]. There are very few studies that evaluated the association of AHSCT applied to patients with MM in Turkey with age. Our objective in this study is to compare the outcomes of patients with MM over 65 years of age who had AHSCT with the outcomes of patients under 65 years of age, based on single-center data. In addition to this, our objective is to evaluate transplantation-associated toxicity and success at 65 years of age.
Materials and Methods
Records on diagnosis, stage, and treatment before and after transplantation of 29 patients over 65 years of age and 39 patients under 65 years of age for whom AHSCT was applied due to diagnosis of MM in the Ankara Numune Training and Research Hospital Bone Marrow Transplantation Unit between 2008 and 2017 were collected. Outcomes of patients’ initial transplantations were evaluated. Our study was approved by Research Ethics Committee, Ankara Numune Training and Research Hospital (E-18-2209-2018). Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards
Patients who had another malignant disease and patients who had received treatment due to diagnosis of MM but had not had transplantation were excluded from the study.
As the transplantation protocol, patients under 65 years of age were administered 200 mg/m2/day and those over 65 years of age were administered 140 mg/m2/day of melphalan infusion for at least 20 minutes. During administration of melphalan, no infusion reaction was observed. All of the patients received antimicrobial prophylaxis with acyclovir, fluconazole, and ciprofloxacin. Patients were initiated with 5 µg(micrograms)/kg of filgrastim treatment 5 days after stem cell transplantation; it was continued for 3 more days after neutrophil count reached >0.5 × 109/L and then it was discontinued. Patients’ daily complete blood counts, renal and hepatic function tests, and electrolytes were recorded. When the temperature of the patients was 38 °C or above, blood cultures were taken and then broad-spectrum antibiotics were initiated. Erythrocyte replacement was provided when a patient’s hemoglobin value was <8 g/dL and thrombocyte suspension was administered when thrombocyte count was below 20 × 109/L.
In the procedure for collecting patients’ stem cells, after treatment with high-dose cyclophosphamide (2-4 g/m2), 10 µg/kg/day of filgrastim was administered from day 3 to the time of leukapheresis. When the leukocyte count of the patients reached 1-2 × 109/L, stem cell collection was performed in the apheresis unit.
The treatments of the patients applied before transplantation and the response criteria used during follow-up after transplantation were defined as follows:
Complete remission (CR): Negative immunofixation in serum and urine, less than 5% plasma cells in bone marrow, lack of soft tissue plasmacytomas.
Very Good Partial Response (VGPR): M protein in serum and urine, which is absent in electrophoresis but can be detected in immunofixation or reduction in serum M protein by 90% or more and <100 mg/24 hours urine M protein.
Partial Response (PR): Reduction in serum M protein by 50% and reduction in 24-hour urine M protein by 90% or decreasing to less than 200 mg/24 hours; if serum or urine M protein cannot be measured, reduction in the difference between free light chains by 50%, instead of M protein criterion; if serum or urine M protein cannot be measured and it cannot be measured in serum light chain, reduction in plasma cells by 50% and, if present in the beginning, reduction in soft tissue plasmacytomas by 50%, instead of iv-M protein, on the condition that the basal ratio of bone marrow plasma cells is at least 30%.
Stable Disease (SD): Defined as disease that did not meet the criteria for complete remission, very good partial response, and progressive disease.
Progressive Disease (PD): With a 25% increase in any of the following compared to the deepest response obtained, it was defined as 1) absolute increase ≥0.5 g/dL in serum M-component; 2) absolute increase of ≥200 mg/24 hours in urine M-component; 3) for bone marrow plasma cell percentage, absolute count >10%; 4) development of new bone lesions or soft tissue plasmacytomas or increase of size of preexisting bone lesions and soft tissue plasmacytomas, 5) development of hypercalcemia, which can be attributable to plasma cell disease (corrected serum calcium >11.5 mg/dL).
Statistical analysis
Statistical evaluation was performed using SPSS 20 for Windows (IBM SPSS Inc., Armonk, NY, USA). Normal distribution of the data was evaluated using the Kolmogorov-Smirnov test. Numerical variables exhibiting normal distribution were represented as mean±standard deviation; numerical variables not exhibiting normal distribution were represented as median (min-max). Categorical variables were given as count and percentage. Between two groups, the Student t-test was used for comparison of numerical variables exhibiting normal distribution and the Mann-Whitney U test was used for numerical variables that did not exhibit normal distribution. For comparison of categorical data the chi-square and Fisher exact chi-square tests were used. Survival evaluation between age groups was performed using Kaplan-Meier analysis.
In statistical analyses, p<0.05 (*) was considered to be significant.
Results
Demographical characteristics of the patients are provided in Table 1. The rate of chronic obstructive pulmonary disease (COPD) was determined to be higher in patients at and over 65 years of age compared to patients under 65 years of age (20.7% versus 2.6%; p=0.042), while distributions of other comorbidities did not differ significantly (p>0.05).
Mean hemoglobin was determined to be lower in patients at and over 65 years of age compared to patients under 65 years of age (9.8±2.0 versus 11.1±2.3; p=0.013). Other laboratory findings are provided in Table 2 in detail.
Clinical characteristics of the patients are provided in Table 3. The ratio of patients in whom cytogenetic anomalies were positive was determined to be higher in patients at and over 65 years of age compared to patients under 65 years of age (24.1% versus 5.1%; p=0.036). All of the patients who were determined to have 17p deletion, 13q deletion, and T(4;14) cytogenetic anomaly were in the older age group. The ratio of the patients in whom a complex cytogenetic anomaly was determined was higher among patients at and over 65 years of age compared to patients under 65 years of age (3.4% versus 2.6%; p=0.017).
First-line and initial AHSCT outcomes of the patients are represented in Table 4. Accordingly, the ratio of patients who received bortezomib treatment as the first-line treatment and response distributions before AHSCT did not differ significantly between patients at and over 65 years of age and patients under 65 years of age (p>0.05). The ratio of patients who developed mucositis in AHSCT (65.5% versus 20.5%; p<0.001) and the ratio of patients who developed neutropenic fever (62.1% versus 33.3%; p=0.027) were determined to be higher in patients at and over 65 years of age compared to patients under 65 years of age. The ratio of patients whose initial AHSCT response at 3 months to 1 year was PR or less was determined to be higher in patients at and over 65 years of age compared to patients under 65 years of age (p<0.05).
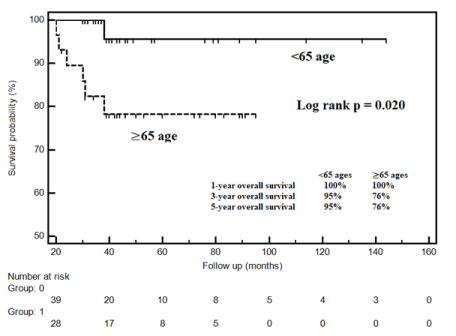
All of the patients with progression died. While mean survival time in patients at and over 65 years of age was 80.4±5.3 months (95% CI: 69.9-90.8), mean survival time in patients under 65 years of age was 139.4±4.5 months (95% CI: 130.6-148.2). While the 5-year survival rate in patients at and over 65 years of age was 76%, it was 95% in patients under 65 years of age (log rank p=0.020) (Figure 1).
Discussion
The success of recently emerged treatments for MM has brought into question AHSCT’s application in advanced age patients with MM. Advanced age poses a problem not only for transplantation but also for intensive chemotherapies. Our objective in this study was the retrospective evaluation of whether the successful transplantation outcomes known in younger patients are similarly successful in patients over 65 years of age despite transplantation regimens applied with lower doses and with the complications for patients over 65 years of age taken into consideration.
In our study, stage, sex, and response rate to induction chemotherapy of both groups were observed to be similar. Age and sex distributions in our study showed parallelism with outcomes of transplantation studies involving patients with MM in this age group (median age: 52 and 67 years) [12].
The Durie-Salmon (DS) and International Staging System (ISS) scores, which are other factors that may influence treatment-transplantation outcome, were similar in both groups. Pre-transplantation beta-2 microglobulin level was determined to be higher in patients over 65 years of age. In other similar studies, beta-2 microglobulin levels have been determined to be higher in older populations [13]. It was thought that this elevation may be associated with decreased creatinine clearance in patients over 65 years of age.
In studies on MM, confirmed presence of a cytogenetic anomaly has been defined as a poor prognostic marker [14]. In studies of older MM patients, the incidence of cytogenetic anomalies has been determined to be higher, and this was associated with the differences in treatments that patients had previously received [15]. Also in our study, the presence of cytogenetic anomalies was determined to be more common among patients over 65 years of age. However, the patients in our study were only those who had received induction treatment and those whose response rates were similar for both groups. Furthermore, it was thought that, independently of the treatment, the presence of cytogenetic anomalies in high rates that was determined with advancing age may have contributed to unfavorable post-transplantation outcomes in this age group.
Although in the IFM 99-0 study, which compared transplantation treatments with melphalan-prednisolone-thalidomide (MPT) versus moderate-dose melphalan in patients with MM aged between 65 and 75 years, MPT was shown to be superior [16], many studies other than that one emphasized that AHSCT is superior in this age group [17,18]. However, the cut-off value for age range was taken as 65-70 years in studies emphasizing that AHSCT was superior [19,20]. In the study conducted by Palumbo et al., [20,21], in which AHSCT was compared with conventional chemotherapies, contrary to what is commonly believed, the superiority of AHSCT applied with low-dose melphalan (100 mg) compared to conventional chemotherapies in younger and older patients (mean: 54-64 years) was emphasized. Outcomes of this study demonstrate that the clinical benefit and the acceptable adverse effects obtained with AHSCT are superior compared to conventional chemotherapies [21]. In many studies in which melphalan was administered at a dose of 140 mg, less toxicity was encountered compared to 200 mg. In our study, the patients received 140 mg of melphalan and our outcomes showed parallelism with those of other studies. In addition to this, although dose of melphalan was decreased during the phase of transplantation in older patients, neutropenic fever and mucositis were more commonly seen compared to the younger age group. This high rate, which was determined despite the reduction of the dose of melphalan, was thought to be associated with the high comorbidity rate (such as COPD) of the patients over 65 years of age in addition to age. In conclusion, 140 mg of melphalan has less toxicity compared to 200 mg in the advanced age MM patient group, and transplantation outcomes were obtained similar to those with 200 mg of melphalan, as well [22]. In our study, response rates below partial remission were determined to be more common among the patients over 65 years of age in a 1-year response evaluation that was performed at 3-month intervals.
According to analysis of international bone marrow transplantation data, 2-year survival was reported to be 84% for all patients [19]. In our study, survival analysis results were determined to be superior to those of other studies. We suggest that the fact that the mean age of our advanced age patients was 66 years contributed to this situation. Furthermore, initial 1-year response evaluations of the older age group were determined to be unfavorable compared to the other group. This situation was thought to be associated with the unfavorable cytogenetic markers the patients had and the lower dose of melphalan administered.
Limitations of this study are determined to be inclusion of only patients aged between 65 and 70 for the advanced age group, the lack of a patient group to be compared with conventional chemotherapies, being a retrospective study, the limited number of patients, and having data obtained from a single center.
In conclusion, AHSCT is still seen as an effective treatment option for patients with MM over 65 years of age. However, studies that are prospective and multi-center, with larger numbers of patients and comparisons of AHSCT with newly emerged agents and targeted agents, are needed for this patient group.
- Tuchman SA, Shapiro GR, Ershler WB, Badros A, Cohen HJ, Dispenzieri A, et al. (2014) Multiple myeloma in the very old: an IASIA conference report. J Natl Cancer Inst 106: 10. Link: https://bit.ly/2LYNUYf
- Palumbo A, Bringhen S, Bertola A, Cavallo F, Falco P, Massaia M, et al. (2004) Multiple myeloma: comparison of two dose-intensive melphalan regimens (100 vs 200 mg/m2). Leukemia 18: 133–138. Link: https://bit.ly/2YzzRdO
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. (1996) A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med 335: 91–97. Link: https://bit.ly/2KcQCHO
- Palumbo A, Cavallo F (2012) Review article have drug combinations supplanted stem cell transplantation in myeloma? Blood. 120: 4692–4698. Link: https://bit.ly/2YmTV7W
- Harousseau J (2008) Symposium article autologous transplantation for multiple myeloma. Ann Oncol 7: 128–133. Link: https://bit.ly/3381Btd
- Winn AN, Shah GL, Cohen JT, Lin PJ, Parsons SK (2015) The real world effectiveness of hematopoietic transplant among elderly individuals with multiple myeloma. J Natl Cancer Inst 107: 1–6. Link: https://bit.ly/2M30cPJ
- El Cheikh J, Kfoury E, Calmels B, Lemarie C, Stoppa AM, et al. (2011) Age at transplantation and outcome after autologous stem cell transplantation in elderly patients with multiple myeloma. Hematol Oncol Stem Cell Ther 4: 30–36. Link: https://bit.ly/2YgN0Nw
- Cavo M, Rajkumar SV, Palumbo A, Moreau P, Orlowski R, et al. (2016) International myeloma working group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood 117: 6063–6073. Link: https://bit.ly/2Kx2ovF
- Brenner H, Gondos A, Pulte D (2008) Recent major improvement in long term survival of younger patients with multiple myeloma. 111: 2521-2526. Link: https://bit.ly/2K9d2cK
- Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, et al. ( 2014) Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 28: 1122–1128. Link: https://bit.ly/2Kjrq0Z
- Sharma SK, Choudhary D, Gupta N, Dhamija M, Khandelwal V, et al. (2014) Cost of hematopoietic stem cell transplantation in India. Mediterr J Hematol Infect Dis Link: https://bit.ly/2SYFjFP
- Powles R, Raje N, Milan S, Millar B, Shepherd V, et al. (1997) Outcome assessment of a population-based group of 195 unselected myeloma patients under 70 years of age offered intensive treatment. Bone Marrow Transplant 20: 435–443. Link: https://bit.ly/2KpHpLc
- Pasqualetti P, Collacciani A, Maccarone C, Casale R (1996) Prognostic factors in multiple myeloma: selection using Cox's proportional hazard model. Biomedical Pharmacotherapy 50: 29-35. Link: https://bit.ly/2yuVIsj
- Desikan R, Barlogie B, Sawyer J, Ayers D, Tricot G, et al. (2000) Results of high-dose therapy for 1000 patients with multiple myeloma: durable complete remissions and superior survival in the absence of chromosome 13 abnormalities. Blood 95: 4008-4010. Link: https://bit.ly/2Ox7RYh
- Badros A, Barlogie B, Siegel E, Morris C, Desikan R, et al. (2001) Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. British Journal of Haematology 114: 600-607. Link: https://bit.ly/2T0Hys9
- Jagannath S, Vesole DH, Zhang M, Desikan KR, Copeland N, et al. (1997) Feasibility and costeffectiveness of outpatient autotransplants in multiple myeloma. Bone Marrow Transplant 20: 445–450. Link: https://bit.ly/33accnR
- Ramiah V, Powles R, Sumpter K (1997) A randomised trial of short course consolidation chemotherapy (MRC UKALL X) following high-dose chemotherapy in multiple myeloma. Blood 90 (Suppl.): 407b.
- Smith H, Kark JD, Cassel JC, Sprears GFS (1977) Analysis of prospective epidemiologic studies by minimum distance casecontrol matching. Am J Epidemiol 105: 567–574. Link: https://bit.ly/338lY9G
- Sirohi B, Powles R, Treleaven J, Mainwaring P, Kulkarni S, et al. (2000) The role of autologous transplantation in patients with multiple myeloma aged 65 years and over. Bone Marrow Transplantation 25: 533–539. Link: https://bit.ly/2YAARCf
- Palumbo A, Triolo S, Argentino C, Bringhen S, Dominietto A, et al. (1998) Melphalan at 100 mg/m2 with stem cell support is superior to standard treatment in multiple myeloma: a retrospective case-matched analysis. Blood Link: https://bit.ly/2YziWff
- Henon P, Donatini B, Eisenmann JC, Becker M, Beck-Wirth G, et al. (1995) Comparative survival, quality of life and cost effectiveness of intensive therapy with autologous blood stem cell transplantation or conventional chemotherapy in multiple myeloma. Bone Marrow Transplant 16: 19-25. Link: https://bit.ly/2YmztDY
- Palumbo A, Triolo S, Argentino C, Bringhen S, Dominietto A, et al. (1999) Dose-intensive melphalan with stem cell support (MEL100) is superior to standard treatment in elderly myeloma patients. Blood 94: 1248-53 Link: https://bit.ly/2yub400
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
