Studies on Stem Cells Research and Therapy
Adipogenic and Osteogenic Markers Characterization of Human Amniotic Fluid Stem Cells
Hassan IH El-Sayyad1*, Mohamed A Sobh2, Soad A Khalifa2, Omnia KRA El-Sayyad3
2MERC & Urology & Nephrology Center, Faculty of Medicine, Mansoura University, Mansoura, Egypt
3Pediatric Hospital, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Cite this as
El-Sayyad HIH, Sobh MA, Khalifa SA, El-Sayyad OKRA (2016) Adipogenic and Osteogenic Markers Characterization of Human Amniotic Fluid Stem Cells. Studies on Stem Cells Research and Therapy 2(1): 025-032. DOI: 10.17352/sscrt.000009Objective: Human amniotic fluid stem cells (HAFSCs) derived from human amniotic fluid during parturition are of good source in regenerative medicine for development to either adipocyte, chondrogenic or osteogenic cells.
Methods and results: The HAFSCs were obtained from amniotic fluid post-parturition. A written informed consent had been taken beforehand from mothers after parturition during admission in Mansoura University Hospital, Egypt. Cultured in adipogenic or osteogenic culture for 4,7,14 & 21 days. Flow cytometry of HAFSCs for Oct-4, CD13,, CD29 , CD 90 , CD34 and CD 14. Gene expression for adipogenic (PCR of leptin, peroxisome proliferator-activated receptor-γ and lipoprotein lipase) and osteogenic (osteocalcin) cells were carried out. Biochemical assessments of adipogenic (lipoprotein lipase enzyme and glycerol-3-phosphate dehydrogenase) and osteogenic (alkaline phosphatase, B-galactosidase and calcium content) markers. HAFSCs were positive for Oct-4, CD13,, CD29 , CD 90 and negative CD34 and CD 14. Also, light and transmission electron microscopic investigation of adipocyte stem cell culture were investigated at 4,7,14 & 21 days in both two models. Adipocyte derived from HASCs displayed fibroblastic morphology and confluence at 7 days and flat-shape with positive oil red staining at 14 &21 days. At ultrastructural level, the adipocyte derived from HASCs exhibited ideal structure. Also, it showed adipogenic gene expression and biochemical investigation. Similar was observed of its osteogenic affinity of bone cells derived from HFASCs.
Conclusion: The authors concluded that HAFSCs are capable of differentiating into adipocyte, osteoblast and chondroblast depending on the culture medium and are important in regenerating medicine.
Abbreviations
Human amniotic fluid stem cells, characterization, Light and transmission electron microscopy, PCR and biochemical charcterization, adipocyte & osteogenic stem cells.
Introduction
Amniotic fluid is a protective, nourishing fluid that surrounds the fetus during intra-uterine life [1]. The amniotic fluid forms during first and mid-trimester with the aid of active transport of sodium and chloride. This facilitated permeability of water across the chorio-amniotic membrane [2]. Amniotic fluid stem cells are separwated from discarded amniotic fluid at termination of gestation and represent a novel source of stem cells for modeling of human genetic diseases [3] and regenerative medicine [4]. Amniotic fluid represent a good source of fetal stem cells, which are derived from mesoderm, ectoderm and definitive endoderm. The HAFCs cells was carried out by c-Kit (CD117). The cell lines show self-renewal capacity reaching 2 fold increase at 36 hours, and, maintain its telomere length at 250 doublings [5,6].
The hAFSCs cells have immunosuppressive properties with a great ability to differentiate into adipogenic, osteogenic, myogenic, endothelial, pulmonary, neurogenic, hepatogenic, cardiac, and pancreatic lineages. These cells have the capability in treatment of diseases such as diabetes, bone defects, neural degeneration, blood disorders and heart and kidney disease [7] through its capabilities of differentiating into adipocytes, osteocytes, chondrocytes, myocytes, and neuronal cells [8]. Human amniotic mesenchymal stem cells were positive for CD73, CD90, CD105, and CD44 and negative for CD34, CD14, CD45, and HLA-DR. Also, they have the great potential to express stem cell markers such as Oct3/4, Sox2, Nanog, Klf4, SSEA4, c-myc, vimentin, and nestin [9]. There is a detected calcitonin receptor in proliferating and osteo-differentiated hAFMSCs. in vitro osteogenic capacity of hAFCs revealed upregulation of Runx2, osterix, alkaline phosphatase, collagen I, and osteopontin, with a capacity of osteogenic differentiation [10].
The present study aimed to illustrate the separation, characterization and adipogenic and osteogenic differentiation of hAFMSCs via real time PCR, cytological and biochemical assays.
Materials and Methods
Collection of samples
Mesemchymal stem cell are collected from the human amniotic fluid (AF) of 15 women who underwent a cesarean delivery. A written informed consent had been taken beforehand from mothers after parturition during admission in Mansoura University Hospital, Egypt. During cesarean delivery, the amniotic fluid was aspirated after the uterine muscle was opened. Cells were isolated from the AF samples immediately after collection by centrifugation at 1100 rpm for 5 minutes. The cells were cultured in 35-mm petri dishes containing 30 mL low-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA) containing 100 U/mL of penicillin, 0.1mg/mL of streptomycin, 10 ng/mL of basic fibroblast growth factor, 10 ng/mL of epidermal growth factor (Peprotech, Rocky Hill, NJ, USA), and 20% of fetal bovine serum (Invitrogen). The media was renewed after incubation of the cells at 37 °C with 5% humidified carbon dioxide for 7 days and the non-adhering AF cells were removed.
The media was replaced twice weekly until the cells reached 70% confluence. MSCs were separated after adding 0.25% trypsin and 1 mM EDTA (Invitrogen) for 3 minutes to the media. The MSCs were released, collected and replanted in a split ratio of 3 flasks under the same culture conditions.
Harvesting and culturing of human amniotic mesenchymal stem cells ( hAFMSCs)
The hAFMSCs are cultured in regular media (DMEM, 10% serum and 1% penicillin/streptomycin,Sigma) for 3 weeks according to De Coppi et al., (2007). Flow cytometric analysis of CD markers and transcription factors characteristics of hAFMSCs was carried out.
in vitro assessment of viability, colonization and cd markers of The hAFMSCs
Viability test: It was checked by 0.04% trypan blue exclusion according to Mclimans et al.[11]. The number of viable stem cells/ml was then calculated according to the following equation.
Colony forming units-fibroblast (CFU-F) assay
Fibroblast like colony growth was evaluated at densities of 25 × 106 cells/well. After 7 days, the capability of mesenchymal stem cells to form fibroblast-like colonies was assessed by contrast-phase microscope.
Flow cytometry for cell surface antigen expressions
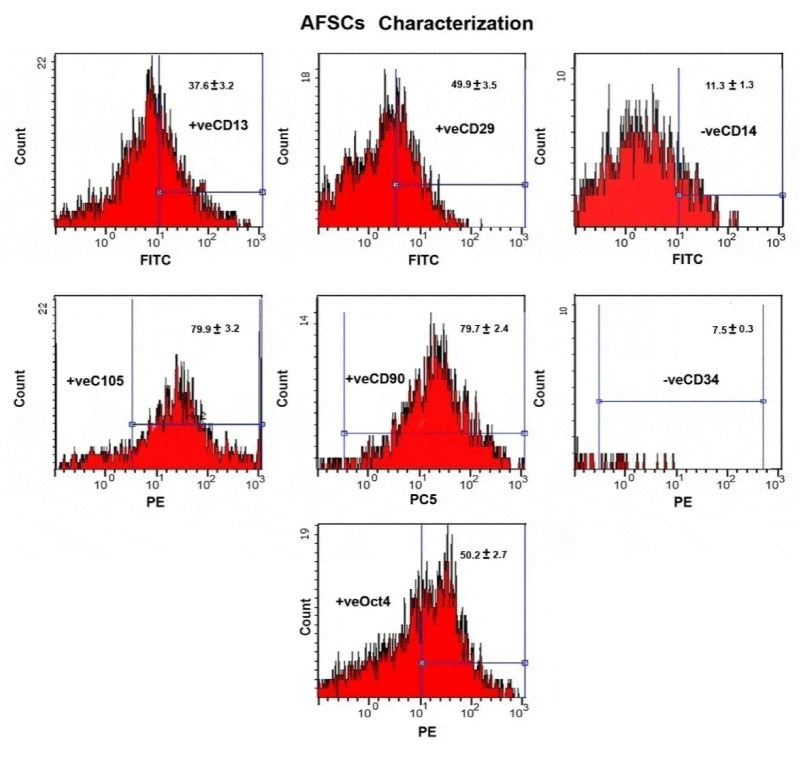
The hAFMSCs were released by trypsinization and assessments of their specific CD markers by flow cytometry. The stem cells at the concentration of (1 x 106 / mL) in phosphate buffer. The monoclonal antibodies used in the different combinations of fluorochromes are fluorescein isothiocyanate (FITC), phycoerythrin (PE) and phycoerythrin-cyanine 5 (PeCY5). Different combinations of mAb were used against various antigens CD14, CD90, CD105, CD13 and CD34.
Osteogenic differentiation
Differentiated hAFMSCs into osteocyte-like cells was carried out, at a density of 1.8X 104 cells /cm2 were cultured in DMEM low-glucose medium containing 10% FBS,1% penicillin/streptomycin (Sigma Chemical Comp. St. Louis, MO, USA), and osteogenic supplement such as dexamethasone (100 nM, Sigma), β-glycerophosphate (10 mM, Sigma), and ascorbic acid-2-phosphate (0.05 mM, Sigma) [12].
Chondrogenic differentiaton
Chondrogenic differentiation was induced by ascorbate, dexamethasone and transforming growth factor according to Zuk et al.[13] and Huang et al [14].
Reverse transcription polymerase chain reaction (RT-PCR):
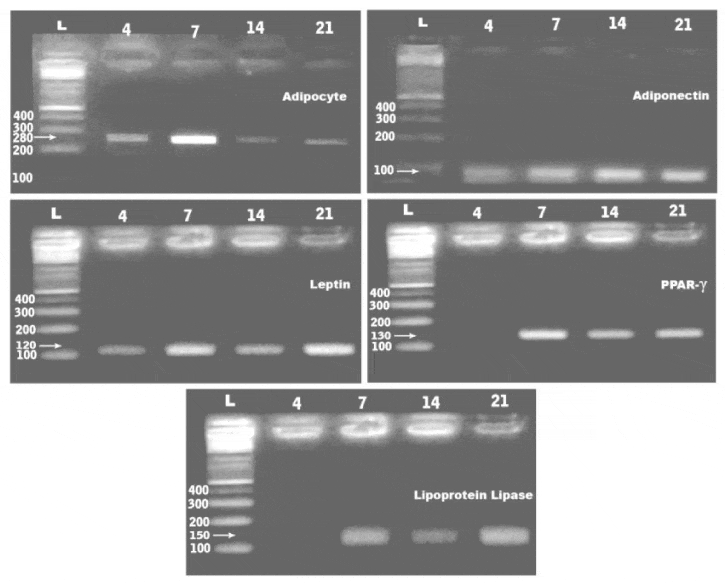
Adipogenic (adipocyte, adiponectin, leptin, lipoprotein lipase & peroxisome proliferator-activated receptor-γ (PPAR- γ) and osteogenic (osteocalcin ) expressed genes were examined differentiated hAFMSCs at 4,7,14 and 21 days. Total RNA was extracted from the cultured cells (QIAGEN Inc., Valencia, CA) and treated with deoxyribonuclease I to remove contaminating genomic DNA. The RT-PCR was carried out using the one step RT-PCR kit. It is started at 50°C for 30 min and 95°C for 15 min for reverse transcription, then followed by 35 cycles, each consisting of denaturation at 94°C for 1 min, annealing at 57°C for 1 min, elongation at 72 °C for 1 min, and the final extension at 72°C for 10 min. The amplified DNA fragments were visualized through 2% agarose gel electrophoreses and photographed under UV light [15] . Reverse transcription was carried out using 1 μg RNA in a total volume of 20 μL.
Real-time PCR was carried out using 25 mL amplification reactions contained primers at 0.5 μm, deoxynucleotide triphosphates (0.2 mm each) in PCR buffer, and 0.03 U Taq polymerase along with SYBR-green (Molecular Probes, Inc., Eugene, OR) at 1:150,000. Aliquots of cDNA were diluted 5- to 5000-fold to generate relative standard curves with cDNA samples [16]. Threshold cycle values of target genes were standardized against GAPDH expression. The primer sequences are illustrated in Tables 1,2). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal standard control.
Biochemical assessments of adipogenic and osteogenic markers
Adipogenic differentiated stem cells were collected, centrifuged and pellet of cells were then disrupted by sonication, and centrifuged at 12,000 × g for 20 min at 4 °C. The supernatants were assayed for lipoprotein lipase (LPL) and glycero-3-phosphate dehydrogenase (GPDH) activities (units/mg protein) using Cell Biolab Elisa Kit according to the manufacturer’s protocol.
In osteogenic differentiated stem cells, alkaline phosphatase (µmoles/cell/hour, catalogue number ABIN2067575) and B-galactosidase activities (Catalogue Number, E1091R) were determined by BALp Elisa kit, Germany. The assay was carried out according to manufacturer’s protocol. Calcium content was determined by colorimetric assay kit of Biovision diagnostic company (USA, cat. Nu, K380-250).
Light microscopic investigation
Adipogenic, osteogenic and chodrogenic stem cells derived from human human amniotic mesenchymal stem cells were stained with either oil red O or alizarin red or saffranin O respectively. The stained cell culture were examined under bright field inverted light microscope and photographed.
Transmission electron microscopic investigation
The adipogenic and osteogenic cell cultured cells at 21 days were centrifuged at 800 g for 10 min and immediately fixed in 2% glutaraldhyde in 0.1 M cacodylate buffer (pH7.4) for 15 min. These was followed by dehydration in ascending grades of ethyl alcohol, cleared in propylene oxide and embedding in epoxy-resin. Ultrathin sections were cut with a diamond knife on a LKB microtome and mounted on grids, stained with uranyl acetate and Lead citrate and examined at 10X Joel Transmission electron microscope.
Statistical analysis
Data were tabulated as mean ± SE. of different cells Statements of significance are based on probability (p) levels of ≤ 0.05 was considered statistically significant. All statistical analyses were undertaken using SPSS program software (version anova).
Results
Flow cytometry characterization and identification of human amniotic fluid stem cells (hAFMSCs)
Human amniotic fluid-derived mesenchymal stem cells (hAFMSCs) have a characterized surface protein expression. Surface protein expression analysis, which allows for a rapid identification of a cell population following flow cytometric analysis of CD markers. Purified amniotic fluid stem cells express high level of Oct-4, CD13, CD29, CD105 and CD90 and these markers were maintained at high levels. Also it is negative for the endothelial and hematopoietic-specific markers CD34 and CD 14 (Figure 1).
Adipogenic stem cells differentiation
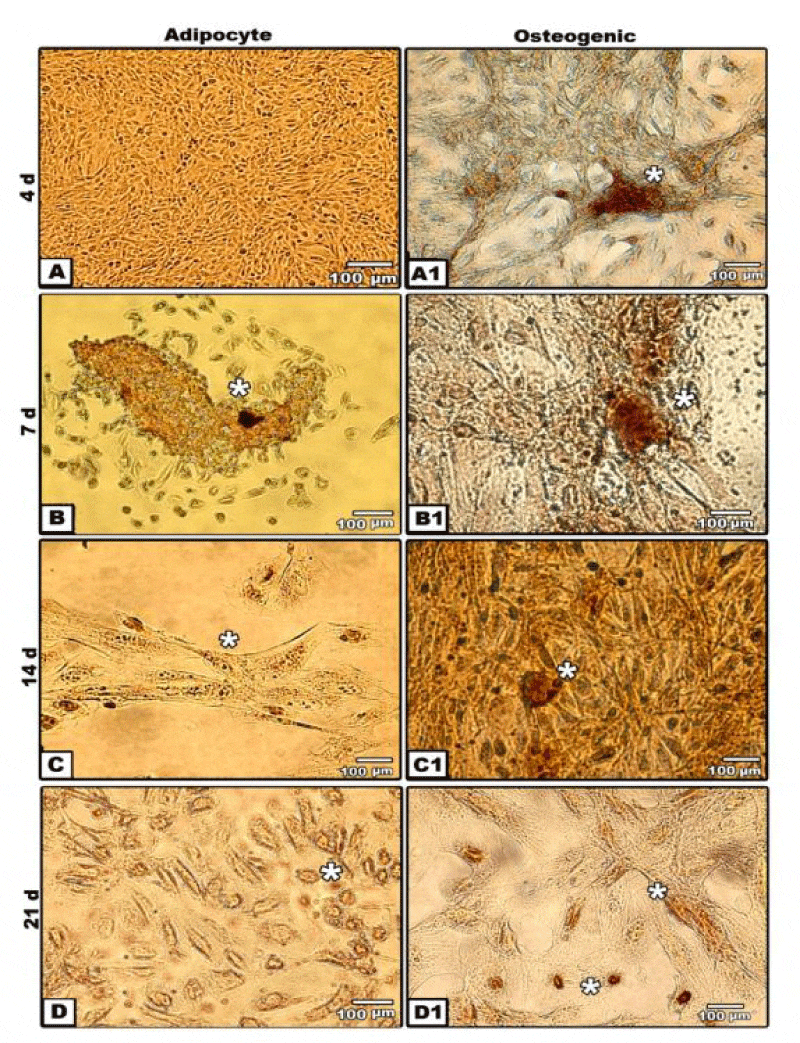
Adipocytes stem cells differentiation from amniotic fluid stem cells firstly, appeared as dedifferentiated and spindle-shaped semi-like fibroblast cells. However, at 4 days, a population of both fibroblastoid and non-fibroblastoid cell types were identified. By 7 days, there is a marked increase fibroblastic activity and increased in its confluency. At 14 and 21 days, the cells acquired flat-shapes with positive oil red staining (Figure 2A,D).
Osteogenic and chondrogenic stem cell differentiation
For confirming osteogenesis, the in vitro cultures were stained with alizarin red and von kossa stain. Following investigating the alizarin-red staining of 4 days cultured osteogenic stem cells derived from hAFMSCs post-osteogenic induction revealed more expansion and dense reddish-brown staining of nodules in isolated regions. Dense mineralized dark-brown deposits was detected at 7 days of osteogenic induction. The peripheral mesenchymal cells of each nodule become spindle-shaped. There was a detected polygonal osteoblast-like phenotypes with mineralization of their extracellular matrix after 14 days of culture manifesting the early sign of bone formation. After 21 days of culture, there was a marked evidences of newly bone formation with dense calcium deposits and mineralization of the extracellular matrix (Figure 2A-B).
On the other hand, von kossa staining of in vitro culture revealed more expansion of oval- and spindle-shaped osteoblast cell with fine granulated mineralized calcium salts during early culturing materials at 4 days post-osteogenic induction. At 7 days, dark mineralization calcium staining was detected. At 14 and 21 days of culture, expansion was markedly increased and mineralization become more visible (Figure 2A1,D1).
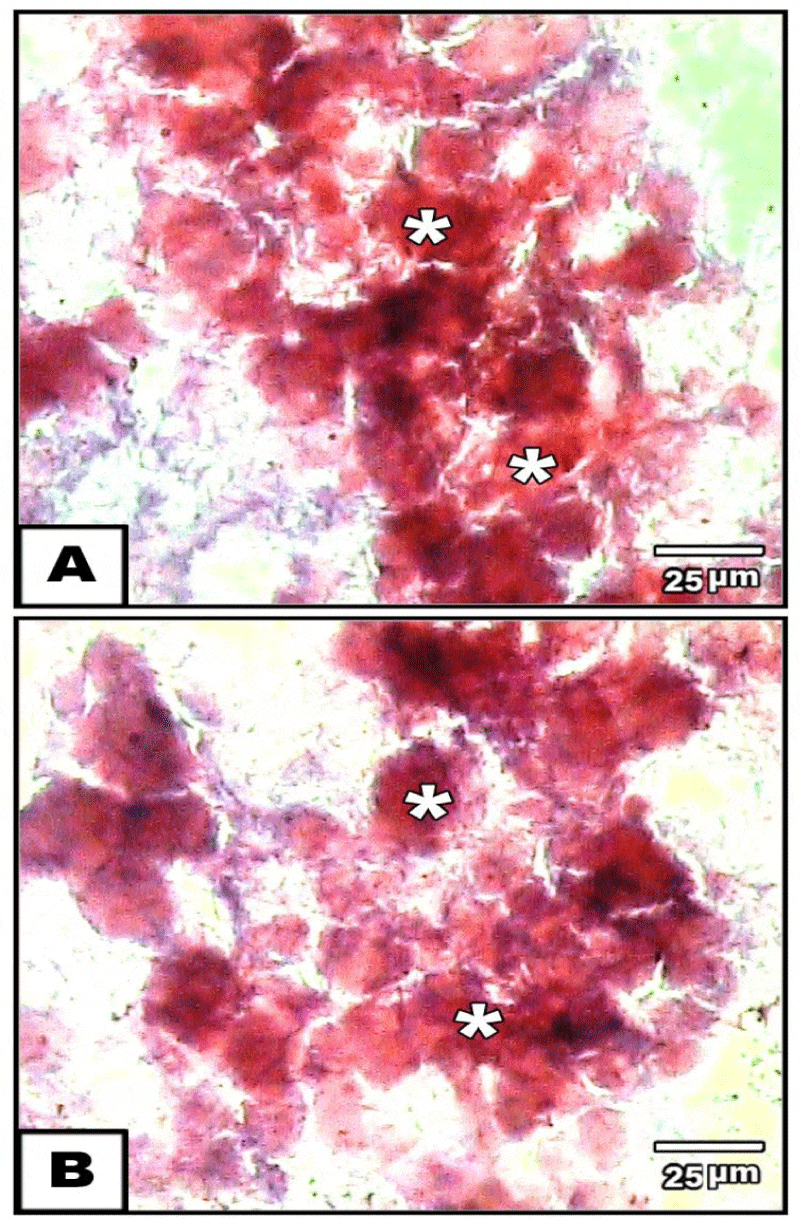
Concerning chondrification, the chondroprogenitor stem cells acquired oval-shaped structure at 21 days post –chondrogenic induction and stained intense red colouration with safranin o (Figure 3A,B).
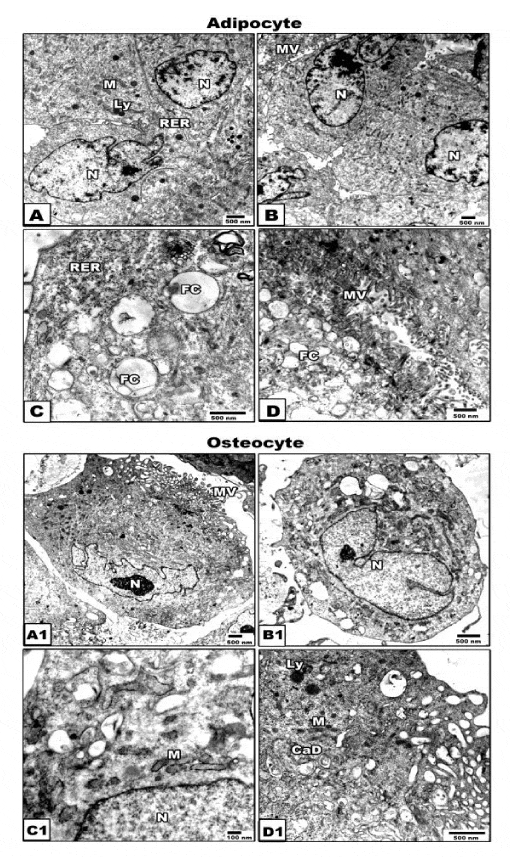
Transmission electron microscopy of adipogenic and osteogenic stem cells at 21 day culture
At ultrastructural level, the adipocyte stem cells exhibited eccentric nucleus, lipid droplet formation, abundant ribosomes, rough endoplasmic reticulum with small cisternae and mitochondria (Figure 4A,D). Concerning the osteogenic cells possessed centrally-located nuclei with either oval or convoluted nuclear envelope. Its cytoplasm contain abundant number of mitochondria and rough endoplasmic reticulum with dilated cisternae. The plasma membrane showed well-developed microvilli. Oval-shaped mineralized calcium deposits become clearly evident. Osteoprogenitor cells revealed the presence of collagen fibrils (Figure 4A1,D1).
PCR identifications of marker genes of adipocyte and osteogenic stem cells
PCR analysis showed expression of the adipocyte, adiponecten, leptin, lipoprotein lipase and peroxisome proliferator-activated receptor-γ (PPAR- γ) at 4,7,14 and 21days of culturing the adipocyte derived stem cells. The gene expression of the assayed genes were 280, 100, 120, 150 and 130 bp respectively (Figure 4). Overexpression of leptin, PPAR-γ and lipoprotein lipase in adipocyte stem cells and osteocalcin in osteogenic stem cells (Table 1). De novo expression of osteocalcin, was more detected at 4 days culture of osteogenic induction comparing with the other in vitro stages (Table 3, Figure 5).
Biochemical confirmation of osteogenesis
To verify osteogenic differentiation of osteogenic stem cells from amniotic fluid-derived mesenchymal stem cells, biochemical determination of alkaline phosphatase and β-galactosidase in osteogenic tissue culture after 21 days of osteogenic induction. Comparing with normal rabbit tibial bone, the osteoblast stem cells reached to 16.58±0.28 comparing with tibia bone of 11.28±0.22. Also, the osteoblast stem cells β -galactosidase reached to 20.37 ± 0.19 comparing with control 15.89±0.3 (Tables 4,5).
Disscussion
From the present findings, hAFMSCs separated from human fluid was firstly appeared as dedifferentiated and spindle-shaped semi-like fibroblast cells and differentiated into both fibroblastoid and non-fibroblastoid cell types by 4 days. Following RT PCR analysis, the observed adipogenic potential of hAFMSCs were observed by increased expression of adiponecten, leptin, lipoprotein lipase and peroxisome proliferator-activated receptor-γ (PPAR- γ) in vitro culture cells at 4,7,14 and 21 days. Similar findings of gene expression of adiponectin [17,18], leptin [19] , lipoprotein lipase [20] and peroxisome proliferator-activated receptor-γ [21] were reported in adipocyte differentiated from hAFMSCs.
Cheong et al. [22] mentioned that cord lining mesenchymal cells differentiate into adipocyte following oil red o staining and presence of lipid droplets and expression of PPARγ and adipsin. Leptin and adiponectin swere extemely low.
Adiponectin is important factor in adipose tissue promoting its proliferation and differentiation from preadipocyte to adipocytes. It affects the expression of CCAAT/enhance binding protein α; perixosome proliferator-activated receptor γ and adipocyte determining and differentiating factor 1/ sterol-regulatory element binding protein-1c [23]. Also it attain marked reduction in type 2 diabetic patients, obesity, or cardiovascular disease [24]. Hypertrophied adipocytes predicted the development of type 2 diabetes [25]. Whereas hyperplasia and hypertrophied of adipocyrtes associated increase burden of inflammatory cells were detected in obese patients.
Leptin is the obese gene, that plays a major role in the in the regulation of adipose homeostasis [26]. Adipocyte-specific cis-regulatory element and the transcription factor(s) responsible for leptin gene expression were recently identified in in vitro adipocyte cultures [27]. Peroxisome proliferator-activated receptor γ (PPARγ) is a nuclear receptor that is a key regulator of adipogenesis and is present in two isoforms generated by alternative splicing, PPARγ1 and PPARγ [28] and enhances lipogenesis, mitochondrial activity, and insulin sensitivity in adipocytes [29]. Lipoprotein lipase expression [20] and leptin expression was found to be increased intracellular lipids storage during adipocyte differentiation [28].
Adipocyte differentiation as confirmed by oil red O staining , gene expression was also confirmed by the bioassays of glycerol -3-phosphate dehydrogenase and lipoprotein lipase enzymes which highly expressed in adipocytes derived from hAFMSCs. The enzyme lipoprotein lipase activity was found to be increased during adipocyte differentiation [20]. Similar glycerol-3-phosphate dehydrogenase had been identified in the control of riglycerides synthesis and/or storage [30] which increased during differentiation of adipocytes [31,32].
Also, transmission electron microscopy of the 21 day of adipocyte cells derived from hAMSCs showed apparent differentiation of cytoplasm lipid droplet and mitochondria. Similar distribution of mitochondria with characterized cristae was reported in adipocyte of human brown adipose tissue [33].
Similar findings of adipogenic differentiation of amniotic fluid-derived stem cells expressing CD117 were reported [34].
Also, the osteogenic stem cells were early recognized at 4 days of in vitro cultures of hAFMSCs stained with alizarine red S. The stem cells form a nodules with peripheral spindle-shaped mesenchymal cells. By 7 days polygonal osteoblast-like phenotype with mineralize their extracellular matrix was observed. By 21 days, there was a detected mineralized calcium deposits in the extracellular matrix. Differentiation of newly formed bone material become more prominent.
Cells belonging to e.g. the amnion, skin, the urogenital, respiratory, and digestive systems have been reported to be detectable. hAFMSCs of CD markers identification, our findings revealed the expression of Octa-4 in human amniotic fluid which represent characteristic type of cells actively divide. These cells were identified by [35] and having the capacity to express cyclin A, a marker for cell cycle proliferation [36].
The observation of the major mineralization events after 21 days of culture is similar with previous studies for mesenchymal stem cells suggesting similar osteogenic potentials [37-39].
Moreover, itt is critical to know that osteocalcin represents one of the important genes responsible for osteoblast and extracellular matrix differentiation. At 21 days of cultured osteogenic cells, osteocalcin, alkaline phosphatase (ALP) and β-galactosidase were remarked. ALP is osteogenic marker and expressed in the process of calcification [40-42]. The extracellular matrix developed vesicles which are believed to arise by budding from the plasma membrane of hard tissue forming cells (osteoblasts, chondrocytes, odontoblasts and cementoblasts) [43].
Similar osteogenesis of cultured hAFMSCs with osteoblast differentiation and overexpression of alkaline phosphatase and mineralized calcium was reported by after implanted the osteogenic materials into an immunodeficient mouse [44,45]. Peister et al. [46], reported osteogenic activity of amniotic fluid stem cells compared to bone morrow-derived stem cells.
Osteogenesis was associated with increased expression of cytoplasmic collagen, osteonectin and bone alkaline phosphatase [47, 468].
Also, osteogenesic differentiation of hAFMSCs into chondrocytes, and osteoblasts, attained much more advanced differentiated process with marked expression of osteogenic markers which need future studies to focus on the mechanical properties of these cells for application in regenerating medicine of healing bone defects. Transmission electron microscopy for the osteogenic cells at 21 days of in vitro culture showed cytoplasm rich in mitochondria, rough endoplasmic reticulum, oval-shaped mineralized calcium deposits and abundant collagen fibrils characteristic for osteoblast differentiatedw cells. Also, cytological pattern of chondrocytes were detected.
Similar findings of in vitro chondrification of mesenchymal stem cells can produce replacement constructs for cartilage defects in osteoarthritis [49] and mouse calvarial mesenchyme [50].
Finally the author concluded that the observations described suggest that hAFMSCs represent a good sources for the isolation of stem cells in regenerative medicine depending on the disease condition.
- Parolini O, Soncini M, Evangelista M, Schmidt D (2009) Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine? Regen Med 4: 275-291. Link: https://goo.gl/FYrpdQ
- Brace RA, Resnik R (1999) Dynamics and disorders of amniotic fluid: maternal Fetal medicine. Philadelphia: Saunders 623-643.
- Ditadi A, de Coppi P, Picone O, Gautreau L, Smati R, et al. (2009) Human and murine amniotic fluid c-Kit+Lin- cells display hematopoietic activity. Blood 113:3953–3960. Link: https://goo.gl/2scs26
- Gholizadeh-Ghaleh Aziz S, Pashaei-Asl F, Fardyazar Z, Pashaiasl M (2016) Isolation, Characterization, Cryopreservation of Human amniotic stem cells and differentiation to osteogenic and adipogenic cells. PLoS One 11: e0158281. Link: https://goo.gl/xHienC
- De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, et al. (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25: 100–106. Link: https://goo.gl/iOxnNo
- Cananzi M, De Coppi P (2012) CD117(+) amniotic fluid stem cells: state of the art and future perspectives. Organogenesis 8: 77-88. Link: https://goo.gl/jlGkj3
- Murphy SV, Atala A (2013) Amniotic fluid and placental membranes: unexpected sources of highly multipotent cells. Semin Reprod Med 31: 62-68. Link: https://goo.gl/cAhUzm
- Savickiene, J, Treigyte G, Baronaite S, Valiuliene G, Kaupinis A, et al. (2015) Human Amniotic Fluid Mesenchymal Stem Cells from Second- and Third-Trimester Amniocentesis: Differentiation Potential, Molecular Signature, and Proteome Analysis. Stem Cells Int 2015: 319238. Link: https://goo.gl/D66Viu
- Teng Z, Yoshida T, Okabe M, Toda A, Higuchi O, et al. (2013) Establishment of immortalized human amniotic mesenchymal stem cells. Cell Transplant 22: 267-78. Link: https://goo.gl/1xiLD0
- Si J, Dai J, Zhang J, Liu S, Gu J, et al. (2015) Comparative Investigation of Human Amniotic Epithelial Cells and Mesenchymal Stem Cells for Application in Bone Tissue Engineering. Stem Cells Int 2015: 565732. Link: https://goo.gl/TMvXVP
- McLimans WF, Davis EV, Glover FL, Rake GW (1957) The submerged culture of mammalian cells; the spinner culture. J Immunol 9: 428–433. Link: https://goo.gl/nQGRdP
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP (1997) Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem 64: 295-312. Link: https://goo.gl/MNqDEG
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-228. Link: https://goo.gl/bCA69X
- Huang CY, Hagar KL, Frost LE, Sun Y, Cheung HS (2004) Effects of cyclic compressive loading on chondrogenesis of rabbit bone- marrow derived mesenchymalstem cells. Stem Cells 22: 313-323. Link: https://goo.gl/vYLWDX
- Peng H, Zhang X, Du ZW, Zhang JW (2007) Expression of human HZF1 in E. coli and preparation of antibody against human HZF1 protein. Zhongguo. Yi. Xue. Ke. Xue. Yuan. Xue. Bao 29: 772-776. Link: https://goo.gl/NiKHjD
- Rubin C, Esteban E, Kieszak S, Hill RH, Dunlop B, et al. (2002) Assessment of human exposure and human health effects after indoor application of methyl parathion in Lorain County, Ohio, 1995-1996. Environ Health Perspect 110: 1047-1051. Link: https://goo.gl/iK6CEH
- Corbetta S, Bulfamante G, Cortelazzi D, Barresi V, Cetin I (2005) Adiponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab 90: 2397–2402. Link: https://goo.gl/U8AICg
- Sobh MA, El-Sayyad HIH, Khalifa SA, Bakr EH, El-Sayyad OKR (2013) in vitro comparative studies of adipocyte stem cells derived from human tissue and amniotic fluid cells. Int. J Exp Pharmacol 4: 29-34. Link: https://goo.gl/aG1r7K
- De Gemmis P, Lapucci C, Bertelli M, Tognetto A, Fanin E, et al. (2006) A real-time PCR approach to evaluate adipogenic potential of amniotic fluid-derived human mesenchymal stem cells. Stem Cells Dev 15: 719-728. Link: https://goo.gl/leLUec
- Gonzales AM, Orlando RA (2007) Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr Metab 30: 4-22. Link: https://goo.gl/neM9iU
- Takenaka Y, Inoue I, Nakano T, Shinoda Y, Ikeda M, et al. (2013) A Novel Splicing Variant of Peroxisome Proliferator-Activated Receptor-γ (Pparγ1sv) Cooperatively Regulates Adipocyte Differentiation with Pparγ2. PLoS ONE 8: e65583. Link: https://goo.gl/Iyocrd
- Cheong HH, Masilamani J, Phan TT, Chan SY (2010) Cord lining progenitor cells: potential in vitro adipogenesis model. Int J Obes (Lond) 34: 1625-1633. Link: https://goo.gl/UJ0yo5
- Fu Y, Luo N, Klein RL, Carvey WT (2005) Adiponectin promotes adipocyte differentiation. Insulin sensitivity and lipid accumulation. J Lipid Res 46: 1369–1379. Link: https://goo.gl/CtvyqR
- Havel P (2002) Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 13: 51–59. Link: https://goo.gl/6z1h41
- Lonn M, Mehlig K, Bengtsson C, Lissner L (2010) Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J 24: 326–331. Link: https://goo.gl/whalRe
- Hawang CS, Loftus TM, Mandrup S, Lane MD (1997) Adipocyte differentiation and leptin expression. Annu Rev Cell Dev Biol 13: 231- 259. Link: https://goo.gl/ljsQRf
- Wrann CD, Eguchi J, Bozec A, Xu Z, Mikkelsen T, et al. (2012) FOSL2 promotes leptin gene expression in human and mouse adipocytes. J Clin Invest 1223: 1010-1021. Link: https://goo.gl/42JnFQ
- Li W, Xu L, Chen Y, Mu L, Cheng M, et al. (2016) Effect of estrodiol on leptin receptors expression in regulating fat distribution and adipocyte genesis. Gynecol Endocrinol 32: 464-468. Link: https://goo.gl/niRZgR
- Deng T, Sieglaff DH, Zhang A, Lyon CJ, Ayers SD, et al. (2011) A peroxisome proliferator-activated receptor gamma (PPARgamma)/PPARgamma coactivator 1beta autoregulatory loop in adipocyte mitochondrial function. J Biol Chem 286: 30723-30731. Link: https://goo.gl/kHsVEU
- Brown LJ, Koza RA, Marshall L, Kozak LP, MacDonald MJ (2002) Lethal hypoglycemic ketosis and glyceroluria in mice lacking both the mitochondrial and the cytosolic glycerol phosphate dehydrogenases. J Biol Chem 277: 32899-32904. Link: https://goo.gl/eZPR9Z
- Spiegelman BM, Puigserver P, Wu Z (2000) Regulation of adipogenesis and energy balance by PPARgamma and PGC-1. Int J Obes Relat. Metab Disorder 24: 8-10. Link: https://goo.gl/f44cei
- Brown CF, Yan J, Han TT, Marecak DM, Amsden BG, Flyn LE (2015) Effect of decellularized adipose tissue particle size and cell density on adipose-derived stem cell proliferation and adipogenic differentiation in composite methacrylated chondroitin sulphate hydrogels. Biomed Mater 10: 045010. Link: https://goo.gl/RXXvDV
- Liao Y, Zeng Z, Lu F, Dong Z, Chang Q, et al. (2015) In Vivo Dedifferentiation of Adult Adipose Cells. PLoS ONE 10: e0125254. Link: https://goo.gl/wb4Tyv
- Guan T, Chen XL, Wei YJ, Lai Y, Xie LY, et al. (2012) Isolation and biological characterization of human amniotic fluid-derived stem cells. Sichuan Da Xue Xue Bao Yi Xue Ban 43: 15-18. Link: https://goo.gl/9Rf9pN
- Tsai MS, Le JL, Chang YJ, Hwang SM (2004) Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod 19: 1450–1456. Link: https://goo.gl/to9MKC
- Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschlager M (2003) Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod 18: 1489-1493. Link: https://goo.gl/KE4mOS
- Colter DC, Sekiya I, Prockop DJ (2001) Identification of a subpopulation of rapidly selfrenewing and multipotential adult stem cells in colonies of human marrow stromal cells. Nat Acad Sci USA 98: 7841–7845. Link: https://goo.gl/cDL5MP
- Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, et al. (2002) Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orth Reserch 20: 1060–1069. Link: https://goo.gl/p5RHtB
- Zur Nieden NI, Kempka G, Ahr HJ (2003) in vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation 71: 18-27. Link: https://goo.gl/HhBRbw
- Benoit DS, Durney AR, Anseth KS (2006) Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng12: 1663-1673. Link: https://goo.gl/OjXOaX
- El-Amin SF, Botchwey E, Tuli R, Kofron MD, Mesfin A, et al. (2006) Human osteoblast cells: isolation, characterization, and growth on polymers for musculoskeletal tissue engineering. J Biomed Mater Res A 76: 439-449. Link: https://goo.gl/BtfZ4Y
- 42. Chen HN, Fan S, Weng CF (2007) Down-regulation of TGF beta1 and leptin meliorates thioacetamide-induced liver injury in lipopoly saccharide primed rats. J Endotoxin Res 13: 176-188. Link: https://goo.gl/FXjQo4
- Damek-Poprawa M, Golub E, Otis L, Harrison G, Phillips C, et al. (2006) Chondrocytes utilize a cholesterol dependent lipid translocator to externalize phosphatidylserine. Biochemicals 45: 3325–3336. Link: https://goo.gl/TQLj2B
- Guan T, Che XL, Wei YJ, Lai Y, Xie LY, et al (2012) Isolation and biological characterization of human amniotic fluid-derived stem cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 43: 15-18. Link: https://goo.gl/t2BIju
- Wang Y, Yin Y, Jiang F, Chen N (2015) Human amnion mesenchymal stem cells promote proliferation and osteogenic differentiation in human bone marrow mesenchymal stem cells. J Mol Histol 46: 13-20. Link: https://goo.gl/dTjhmk
- Peister A, Woodruff MA, Prince JJ, Gray DP, Hutmacher DW, et al. (2011) Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res 7: 17–27. Link: https://goo.gl/n1Jc6q
- Silva WA, Covas D, Panepucci RA, Proto-Siqueira R, Siufi J,et al (2003) The Profile of Gene Expression of Human Marrow Mesenchymal stem cells. Stem Cells. 21, 661-669. Link: https://goo.gl/OW02vP
- Mygind T, Stiehler M, Baatrup A, Li H, Zou X, et al. (2007) Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 28: 1036-1047. Link: https://goo.gl/Om1bS4
- Chen FH, Tuan RS (2008) Adult stem cells for cartilage tissue engineering and regeneration. Curr Rheumatology Rev 4: 161-170. Link: https://goo.gl/kodLxX
- Aberg T, Rice R, Rice D, Thesleff I, Waltimo-Siren J (2005) Chondrogenic potential of mouse calvarial mesenchyme. J Histoch Cytoch 53: 653-663.. Link: https://goo.gl/QWVJbD
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
