Studies on Stem Cells Research and Therapy
Adipose Derived Mesenchymal Stem Cell Differentiation into Adipogenic and Osteogenic Stem Cells
Hassan IH El-Sayyad1*, Mohamed A Sobh2, Soad A Khalifa2, Omnia KRA El-Sayyad3
2Urology & Nephrology Center, Research Center, Egypt
3Pediatric Mansoura University Hospital, Mansoura University, Egypt
Cite this as
El-Sayyad HIH, Sobh MA, Khalifa SA, El-Sayyad OKRA (2016) Adipose Derived Mesenchymal Stem Cell Differentiation into Adipogenic and Osteogenic Stem Cells. Studies on Stem Cells Research and Therapy 2(1): 025-032. DOI: 10.17352/sscrt.000008Objective: Lipoaspiration of human breast fats are important source of adipocyte stem cells (hAMSCs) which play a great role in regenerative medicine. The present study illustrates its capability of its transformation and characterization of adipocyte, osteogenic or chondrogenic cells.
Methods and results: The hAMSCs were positive for CD13, CD29, CD105 and CD90 and negative CD34 and CD 14. The hAMSCs were cultured in adipogenic or osteogenic culture for 4,7,14 & 21 days. Gene expression for adipogenic (PCR of leptin, peroxisome proliferator-activated receptor-γ and lipoprotein lipase) and osteogenic (osteocalcin) cells were carried out. Biochemical assessments of adipogenic (lipoprotein lipase enzyme and glycerol-3-phosphate dehydrogenase) and osteogenic (alkaline phosphatase, B-galactosidase and calcium content) markers. Also, light and transmission electron microscopic investigation of adipocyte stem cell culture were investigated at 4,7,14 & 21 days in both two models. Adipocyte derived from hAMSCs displayed fibroblastic morphology and confluency at 7 days and flat-shape with positive oil red staining at 14 &21 days. At ultrastructural level, the adipocyte derived from hAMSCs exhibited ideal structure. Also, it showed adipogenic gene expression and biochemical investigation. Similar was observed of its osteogenic affinity of bone cells derived from hAMSCs.
Conclusion: The authors concluded that adipocyte stem cells are capable of differentiating into adipocyte, osteoblast and chondroblast depending on the culture medium and are promising in regenerating medicine.
Introduction
Adipose mesenchymal stem cells (AMSCs s) are sub-population of fibroblasts reside within the fat tissue [1,2] and development from mesoderm [3,4]. It can be differentiated into stem cells such as adipocytes [5], osteoblasts, chondrocytes and myocytes [6]. Adipogenesis is important in renewing of human soft-tissue such as improve contour defects and wrinkles and new blood vessel growth [7,8]. In laboratory, differentiation into adipocytes takes 14 days and the gene involved for its signaling is investigated [9]. In vitro, early characterization of adipogenesis is enhanced by insulin-like growth factor-1 (IGF-1). Growth hormone, glucocorticoids, insulin and fatty acids. Neovascularization was reported in vivo studies of mice and rats post- simultaneously implanted ASCs seeded artificial scaffolds [10].
Leptin, is the marker hormone of obesity and secreted by adipocytes [11]. Its receptor was activated in the bone marrow to inhibit bone formation and enhancing marrow adipogenesis [12] via activating Jak2/Stat3 expression in bone marrow stromal cells [13]. Adiponectin is secreted by adipocytes and play a great role in glucose metabolism energy homeostasis and increased the expression of osteoblast-related genes in hAMSCs, such as osteocalcin, alkaline phosphatase, and runt-related transcription factor-2 in a dose- and time-dependent manner [14]. Adiponectin infusion improved both glucose level and bone regeneration in diet-induced obesity mice [15]. PPARs increased adiponectin gene transcription via the peroxisome proliferator response elements (PPRE) at the adiponectin promoter [16]. Osteocalcin is an osteoblast specific genes [17] present in bone, second only to collagen type I [18] and have great in the differentiation of osteoblast progenitor cells especially in both matrix synthesis and mineralization [19].
Preliminary studies studied the growth kinetics of lipoaspiratecell population (PLA) and mentioned that PLA cells may have multi-lineage cells [20]. The purpose of the present work is to illustrate the separation of stem cells from human breast adipose tissue and investigate its differentiation and characterization into adipocyte, chondrocyte and osteocyte cells via cellular, biochemical and genetic tools.
Materials and Methods
The study was approved by the department of evaluation and quality assurance of the Mansoura University of Human Research Ethics committee, Egypt. A written informed consent had been taken beforehand from all patients to use their lipoaspirate fat tissue after operation.
Adipose tissue samples
Adipose tissue (fat) lipoaspirated during elective plastic surgery procedures (abdominoplasty or breast reduction) at Mansoura University Hospital, Mansoura, Egypt. The lipoaspirate was dissociated, in a sterile container with ambient temperature till the time of harvest within 8h of aspiration. Keeping lipoaspirates in refrigerator was avoided due to solidification at low temperatures.
Harvesting and Culturing of hAMSCs
Processed lipoaspirates (PLA) cells were obtained from raw human fat and cultured [21]. Raw lipoaspirates were washed carefully in phosphate buffered saline (PBS) and hydrolysed by 0.075% collagenase (type I, Sigma) in PBS for 30 minutes at 37 °C and centrifuged for 10 minutes at low speed. The cell pellet was resuspended in DMEM/10% FBS and filtered through a 100 micron mesh filter to remove cell debris. The filtrate was centrifuged andthe cells are incubated at 37ºC in a 5% CO2 humidified incubator.
In vitro assessment of stem cells
Viability test: The viability of stem cells was regularly qualitatively checked by trypan blue according to the method of Mclimans et al. [21] during the intervals of stem cell cultures. These were carried out by mixing equal volume of both solution 0.04% trypan blue and stem cells for 10 minutes at 37oC then checked of viable cells (unstained) by light microscope.
Colony forming units-fibroblast (CFU-F) assay: Fibroblast like colony growth was evaluated on primary cells grown on tissue culture six-well dishes. The cells were plated at the density of 25 × 106 cells/well. After 7 days, the capability of mesenchymal stem cells to form fibroblast-like colonies was assessed. MSCs morphology was examined byconfocal phase-contrast microscope.
Flow cytometry for cell surface antigen expressions
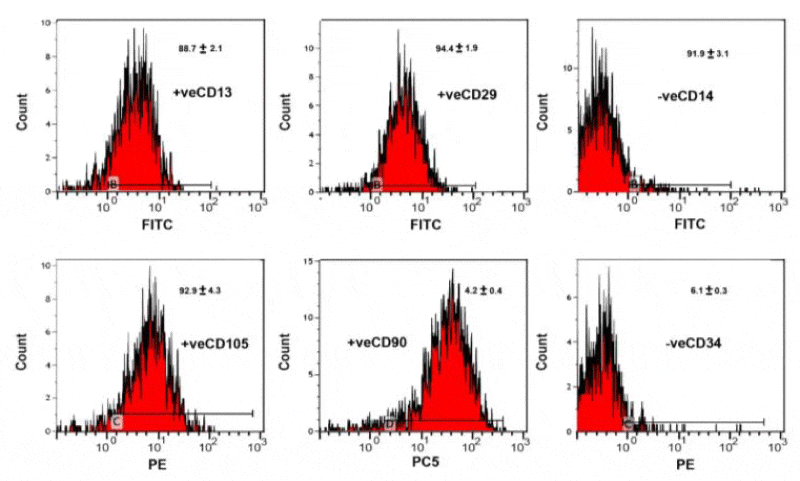
The adipose tissue derived mesenchymal stem cells was released by trypsinization and assessments of their specific CD markers by flow cytometry. The cells were centrifuged at 1200 rpm / 5 minutes and mixed with phosphate buffered saline (PBS) at a concentration of 1 x 106 / mL.100 µL. The cell suspension was conjugated with 10 µLof the fluorescene labeled mAb and incubated in dark room temperature for 30 min. Washing with PBS containing 2% BSA was carried out and the pellet was resuspended in PBS and analyzed immediately on flow cytometry. The monoclonal antibodies used in the different combinations of fluorochromes; namely fluorescein isothiocyanate (FITC), phycoerythrin (PE) and phycoerythrin-cyanine 5 (PeCY5). Different combinations of mAb were used against various antigens CD 14, CD 90, CD 105, CD 13, CD 34. The immunophenotyping was carried out on EPICS-XL flowcytometry (Coulter, Miami, and Fl). The cells were analyzed with the most appropriate gate using the combination of forward and side scatters.
Adipogenicand osteogenic differentiation
Adipose stem cells are seeded at a density of 300,000/ well in a culture dish containing 2 mL PBS. Incubate the cells at 37°C in a 5% CO2 humidified incubator overnight. They are cultured in DMEM low glucose medium (Sigma Chemical Comp. St. Louis, MO, USA) with 10% FBS (Sigma Chemical Comp. St. Louis, MO, USA), 1% penicillin/ streptomycin (Sigma Chemical Comp. St. Louis, MO, USA), and a different adipogenic supplement such as dexamethasone (1 mM, Sigma), 3-isobutyl-1-methylxanthine (1 mM, Sigma), insulin (10 mg/ml, Sigma), and indomethacin (60 mM, Sigma) as adipogenesis induction medium (AIM) and adipogenesis maintenance medium (AMM ) consisting of DMEM low glucose medium (Sigma Chemical Comp. St. Louis, MO, USA) with 10% FBS (Sigma Chemical Comp. St. Louis, MO, USA), 1% penicillin/ streptomycin (Sigma Chemical Comp. St. Louis, MO, USA) and insulin (10 mg/ml, Sigma), as reported by Jaiswal et al. [22].
Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis of different genes
Differentiated adipocytes at 4,7,14 and 21 days derived from lip aspirate were used. Total RNA was extracted by using RNase Minikit (QIAGEN Inc., Valencia, CA) and treated with deoxyribonuclease I to remove genomic DNA. The RT-PCR procedure was carried out using the One Step RT-PCR kit. The amplified DNA fragments were visualized through 2% agarose gel electrophoreses and photographed under UV light [23]. Reverse transcription was carried out using 1 μg RNA in a total volume of 20 μL.
Real-time PCR was done on a Bio-Rad iCycler (Bio-Rad Laboratories, Inc., Hercules, CA). Twenty-five mL amplification reactions was added to primers at 0.5 μm, deoxynucleotide triphosphates (0.2 mm each) in PCR buffer, and 0.03 U Taq polymerase along with SYBR-green (Molecular Probes, Inc., Eugene, OR) at 1:150,000. Aliquots of cDNA were diluted 5- 5000-fold to construct standard curves with which cDNA sample was compared. The procedures repeated four times with different samples. PCR products were normalized for the amount of 18S amplicons in the reverse transcription sample, which was standardized on a dilution curve [24]. The increased PCR reaction products was monitored by measuring the increase in fluorescence intensity caused by the binding of SYBR green to the double stranded DNA that obtained during PCR cycles. Reaction mixtures were set up. Threshold cycle values of target genes were standardized against GAPDH expression. The primer sequences are illustrated in Tables 1,2. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal standard control.
Biochemical assessments of adipogenic and osteogenic markers
Adipogenic differentiated stem cells were collected centrifuged and pellet of cells were then disrupted by sonication, and centrifuged at 12,000 × g for 20 min at 4 °C. The supernatants were assayed for lipoprotein lipase (LPL) and glycero-3-phosphate dehydrogenase (GPDH) activities (units/mg protein) BALP Elisa Kit, Germany, catalogue no. E1091R and E1091R respectively. In osteogenic differentiated stem cells, alkaline phosphatase (µmoles/cell/hour, catalogue number ABIN2067575) and B-galactosidase activities (Catalogue Number, E1091R) were determined by BALP Elisa kit, Germany. The assay was carried out according to manufacturer’s protocol. Calcium content was determined by colorimetric assay kit of Biovision diagnostic company (USA, cat.Nu.K380-250).
Light microscopic investigation
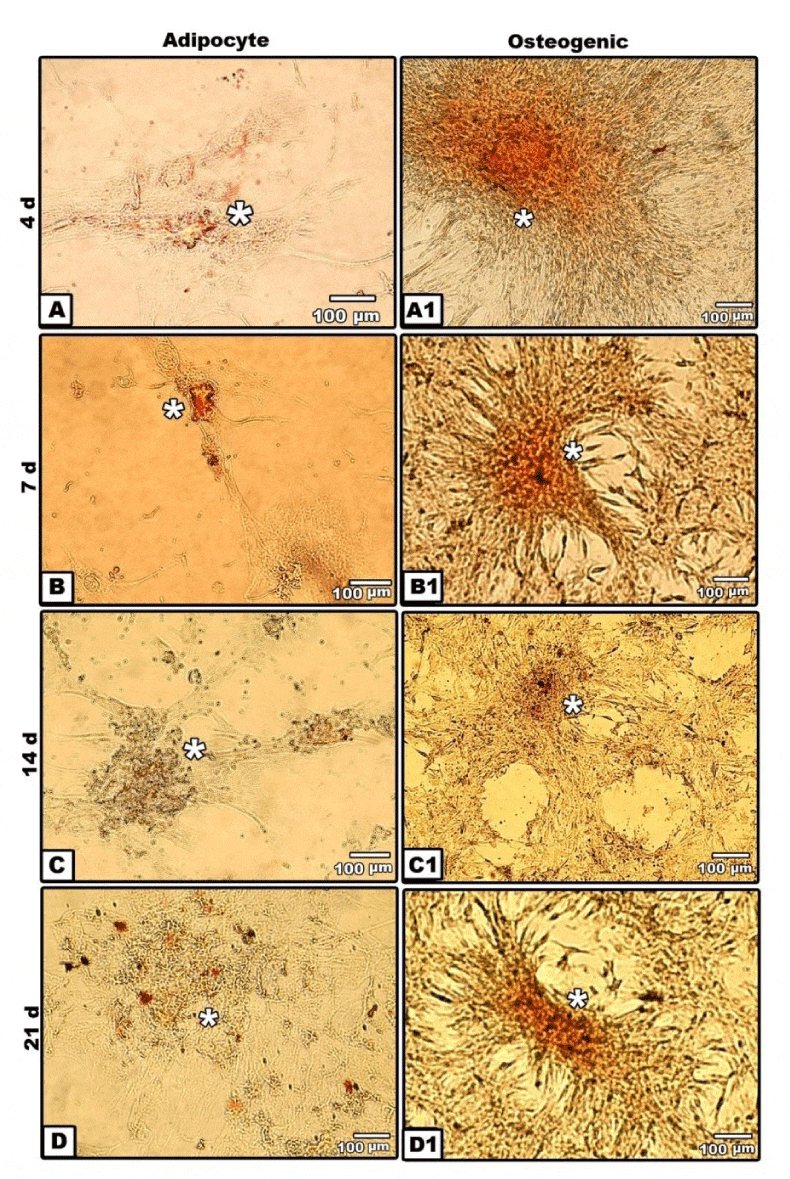
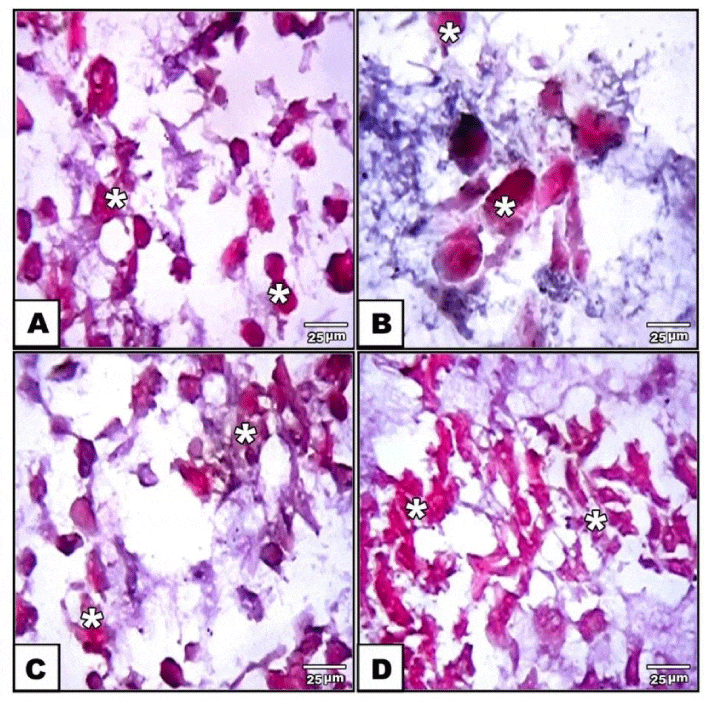
Adipogenic, osteogenic and chondrogenic stem cells derived from human adipose tissue were stained with either oil red O or alizarin red s or safranin O respectively. Then, they were examined and photographed under bright field inverted light microscopy.
Transmission electron microscopic investigation
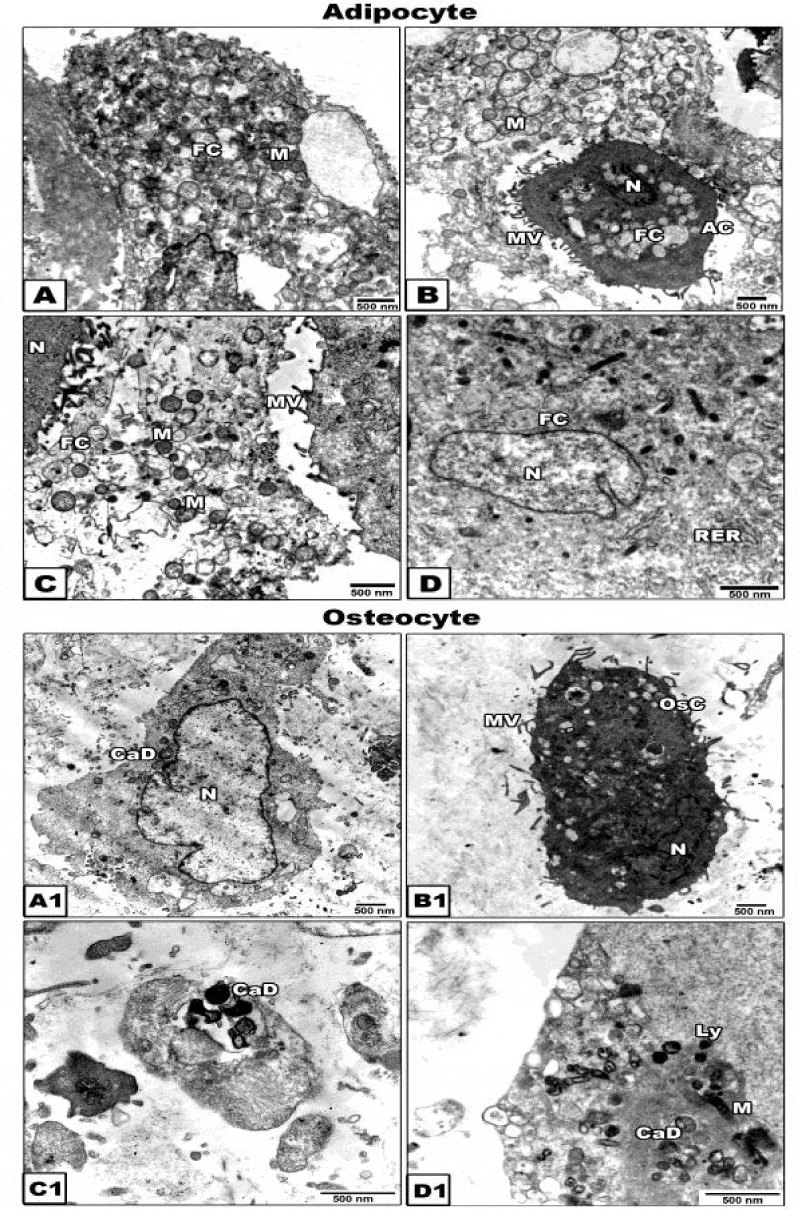
Adipogenic and osteogenic stem cells were collected, centrifuged and pellets of it were immediately fixed in 2% glutaraldhyde in 0.1 M cacodylate buffer (pH 7.4). They were dehydrated in ascending grades of ethyl alcohol and embedded in epoxy-resin. Ultrathin sections were cut with a diamond knife on a LKB microtome, mounted on grids, stained with uranyl acetate and lead citrate and examined at Joel transmission electron microscope 10X at Alexandria Univ. lab.
Statistical analysis
Data were recorded as mean ± SE of different cells. Significance was determined at levels of ≤ 0.05. All statistical analyses were undertaken using multivariance post-hoc analysis of SPSS version 15 program software.
Result
Flow cytometry characterization
Human adipose tissue-derived mesenchymal stem cells have a specific surface protein expression such as positive CD13, CD29, CD105 and CD90 and negative CD34 and CD 14 (Figure1).
Light and transmission electron microscopy of adipogenic stem cell differentiation into adipocytes
Adipogenic tissue was allowed to cultures in non-specific media for two weeks before isolation and culturing in differentiated media. In vitro culture of hAMSCs revealed the establishment of the monolayer cell cultures from the adipose tissue explants. Cells migratedby 7 days of culture; and displayed a fibroblastic morphology. By the end of 7 days culture, clones exhibited substantial heterogeneity and cells with fibroblastic morphology increased in number and confluence (Figure 2B). By 14 and 21 days, the cell morphology changed markedly, from spindle-shaped cells to flat ones. Lipid vesicles could be observed at the end of adipocyte differentiation. The majority of cells in cultures become adipocytes having intracellular accumulation of neutral lipids as detected by oil red staining (Figure 2A-D).
To confirm the morphological criteria of the adipocyte stem cells after 21 days of culture, the cells were examined under transmission electron microscopy. The cells exhibited eccentric nucleus, lipid droplet formation, abundant ribosomes, rough endoplasmic reticulum with small cisternae and mitochondria (Figure 3A1 D1).
Light and transmission electron microscopy of osteogenic and chondrogenic stem cells differentiation from adipocyte stem cells
In vitro cultures of stem cells were stained with alizarin red S. At 4days cultured osteogenic stem cells derived from adipocyte stem cells revealed limited staining of small nodules in isolated regions. The mesenchymal cells take spindle-shaped structures. By 7 days, polygonal osteoblast-like phenotype with mineralize their extracellular matrix was detected. By 21 days, there was a marked evidences of calcium deposits and mineralization in the extracellular matrix (Figure 2A1-D1).
Cytological examination was observed of osteogenic cells at 21 days of In vitro culture. The newly differentiated osteogenic cells is characterized by centrally-located nuclei with convoluted nuclear envelope and cytoplasm rich in mitochondria and rough endoplasmic reticulum with dilated cisternae. The plasma membrane showed well-developed microvilli. Oval-shaped mineralized calcium deposits become clearly evident (Figure 3A1-D1).
PCR identifications of marker genes of adipogenic and osteogenic stem cells
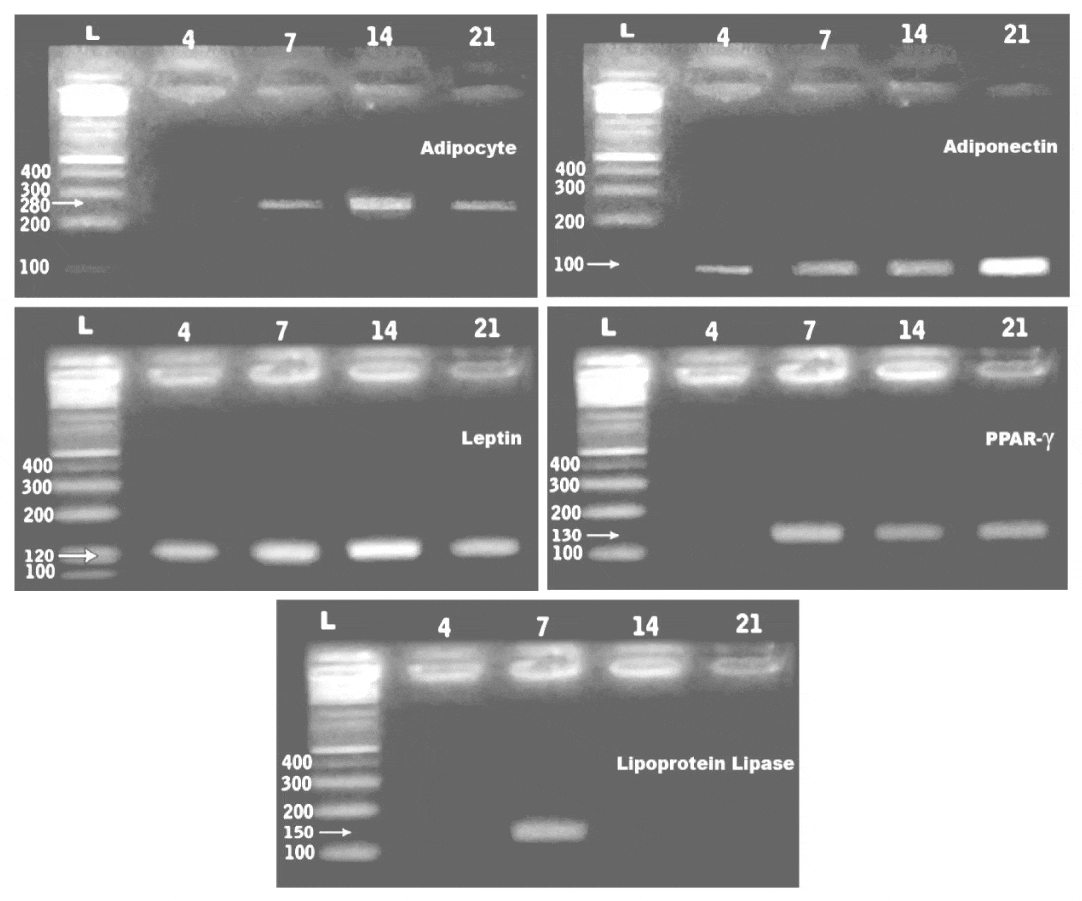
PCR analysis showed expressionof the adipocyte, adiponecten, leptin, lipoprotein lipase and peroxisome proliferator-activated receptor-γ (PPAR- γ) at 4,7,14 and 21days of culturing the adipocyte derived stem cells. The gene expression of the assayed genes were 280, 100, 120, 150and 130 bp respectively (Figure 4).3.3.RT-PCR confirmation of osteocalcin. De novo expression of osteocalcin, was detected at 7day of osteogenic induction compared to the other In vitro stages (Figure 5).
Quantitive Real time PCR of leptin, peroxisome proliferator-activated receptor-γ (PPAR- γ) and lipoprotein lipase
Quantitative real-time PCR analysis was performed using primers specific for leptin, peroxisome proliferator-activated receptor-γ (PPAR- γ) and lipoprotein lipase and GAPDH on a Roche Light Cycler. The assayed genes were carried out with the housekeeping gene GAPDH. The threshold cycle, of a detectable amount of amplicon product has been generated during the early phase of reaction and inversely proportional to the expression level of the studiedgene. Gene expression was determined by real-time PCR after reverse transcription of the applied genes. Quantitation of eptin, peroxisome proliferator-activated receptor-γ (PPAR- γ) and lipoprotein lipase expression in adipocyte stem cells using real time PCR confirmed a detected gene expression compared with the controls. Lepton gene expression reached the lowest level at 4 days of culture and markedly increased at 14 days. However, at 7 and 21 days of adipocyte cell culture, the gene expressed was slightly decreased. However, peroxisome proliferator-activated receptor-γ (PPAR- γ) lacked expression at 4 days of culture and become weakly expressed at the following stages of In vitro culture. Lipoprotein lipase gene expression showed apparent decrease at 4 and 21 days of adipocyte stem cell culture compared with markedly increased at 14 days. ADSCs expressedleptin, peroxisome proliferator-activated receptor-γ (PPAR-γ), the master regulator of adipocyte development, and lipoprotein lipase (LPL) (Table 3).
Biochemical characterization of hAMSCs
Following biochemical determination of lipoprotein lipase enzyme and glycerol-3-phosphate dehydrogenase in adipocyte stem cells after 21 days of culture revealed that marked increase of the expressed biochemical components. Lipoprotein lipase reached to 10.61±0.15.comparing with control human adipose tissue 11.58±0.12 Also, glycerol-3-phosphate dehydrogenase 38.28±0.20 comparing with control human adipose tissue 30.05 ± 0.31 (Table 4).
Also, to verify osteogenic differentiation of osteogenic stem cells from adipocyte-derived stem cells, biochemical determination of alkaline phosphatase and B-galactosidase in osteogenic tissue culture at 21 days of osteogenic induction. Comparing with normal rabbit tibia bone (11.28 ±0.22), the ostoblast stem cells reached to 16.58± 0.28. Also, the osteoblast stem cells B-galactosidase reached to 16.78 ± 0.22 compared to control (15.89±0.3) (Table 5).
Discussion
The field of regenerative medicine is promising of stem cell therapy [25].Adipose tissue is an abundant source of adult/ somatic stem cells for tissue engineering and regenerative medicine [5].
From the present findings, adipocytes stem cells differentiation from lipoaspirates firstly, appeared as dedifferentiated and spindle-shaped semi-like fibroblast cells. However, at 4 days, a population of both fibroblastoid and non-fibroblastoid cell types were identified.
Similar findings were reported following assessments of characteristic morphological features of adipocyte stem cell [26], histochemical staining with oil red o [27] and ultrastructural investigation [28]. Similar dense distribution of mitochondria characterized by tightly backed cristae was reported in adipocytes of human brown adipose tissue [29].
To confirm the adipogenic potential of hAMSCs, marker gene expressions were carried out during adipocyte differentiation by. RT PCR analysis such as adiponecten, leptin, lipoprotein lipase and peroxisome proliferator-activated receptor-γ (PPAR- γ) in In vitro culture cells at 4,7,14 and 21 days. Similar findings of gene expression of adiponectin [30], leptin [31], lipoprotein lipase [32] and peroxisome proliferator-activated receptor-γ [31] were mentioned in adipocyte stem cell differentiation.
Adiponectin is an adipose-secreted protein that exerts both anti-atherogenic and insulin-sensitizing effects [33,34]. The plasma concentration of adiponectin in obese subjects is lower than that in non-obese subjects and inversely correlates with body mass index [35]. Leptin is the obese gene, that plays a major role in the in the regulation of adipose homeostasis [36]. Adipocyte-specific cis-regulatory element and the transcription factor(s) are important for leptin gene expression In vitro adipocyte cultures [37]. Peroxisome proliferator-activated receptor γ (PPARγ) is a nuclear receptor that is a key regulator of adipogenesis and is present in two isoforms generated by alternative splicing, PPARγ1 and PPARγ [31,38] and was found .to enhances lipogenesis, mitochondrial activity, and insulin sensitivity in adipocytes [39]. Lipoprotein lipase expression [32,40] and leptin expression was found to be increased intracellular lipids storage during adipocyte differentiation[31].
The enzyme lipoprotein lipase activity was found to be increased during adipocyte differentiation [41]. Glycerol-3-phosphate dehydrogenase had been identified to control riglycerides synthesis and/or storage in adipocytes [42-44].
Osteogenic differentiation of adipose-derived stem cells started early at 4 days in the form of small nodules of isolated regions with characteristic spindle-shaped cells at its periphery. Von kossa staining of In vitro culture make their first appearance of its dark-brown staining at 7 days and become more visualized by 14 and 21 days of culture. Differentiation of newly formed bone material by staining with alizarin red S stain revealed marked development osteoblasts from hAMSCs. Similar findings of mineralized bone cells were recorded at 21 days of culturing hAMSCs in similar manner [45-47].
These were confirmed by the osteogenic markers including osteocalcin, alkaline phosphatase (ALP) and B-galactosidase. ALP is among the first functional genes expressed in the process of calcification. It is consider as osteogenic marker, as it inevitably leads to mineralization of the neo-tissue [48-50]. ALP is among the first functional genes expressed in the process of calcification. It is consider as osteogenic marker, as it inevitably leads to mineralization of the neotissue [51]. The extracellular matrix developed vesicles which are believed to arise by budding from the plasma membrane of hard tissue forming cells (osteoblasts, chondrocytes, odontoblasts and cementoblasts). hAMSCs can maintain and increase the mineralization for a longer period [52]. Landim de Barros et al. [53], assessed similar osteogenic markers in cultured bone marrow stem cells. The observed patterns of cytoplasmic collagen, osteonectin and bone alkaline phosphatase have been shown to be well-correlated to the osteogenic differentiation process and are involved in the early and intermediate stages of matrix formation and mineralization [47, 54-56].
Moreover, the osteogenic differentiation of adipose derived stem cells exhibit the presence of cacium salts in cultured media following quantitative measurement. Also, hAMS Csdifferentiation into chondrocytes, and osteoblasts was more advanced differentiated process with marked expression of osteogenic markers which need future studies to focus on the mechanical properties of these cells for application in regenerating medicine of healing bone defects as a result of genetic diseases. Transmission electron microscopy for the osteogenic cells at 21 days of osteogenic culture showed almost intact osteogenic progenitor cells characterized by cytoplasm rich in mitochondria, rough endoplasmic reticulum, oval-shaped mineralized calcium deposits and abundant collagen fibrils characteristic for osteoblast differentiated cells.
Similar finding was reported by Tsupykov et al. [57], following assessments of calcium and transmission characterization of a mouse adipose-derived stem/stromal cells and bone marrow stem cells [53].
Also, hAMSCs differentiation into cytological pattern of chondrocytes were detected. Similar findings were reported by Chen and Tuan [58] in patients and Åberg et al. [59], in experimental animals. From this, the hAMSCs are promising in bone therapy for its capacity of expansion and differentiation into osteoblast and chondroblast cells.
Finally the author concluded that the observations described suggest that hAMSCs represents a good sources for the isolation of stem cells used in regenerative medicine.
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, et al. (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301-313. Link: https://goo.gl/P8PAuv
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, et al. (2007) Pericytes of human skeletal muscle are myogenic precursors distinct fromsatellite cells. Nat Cell Biol 9: 255-267. Link: https://goo.gl/2HAB75
- Gonda K, Shigeura T, Sato T, Matsumoto D, Suga H, et al. (2008) Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg 121: 401-410. Link: https://goo.gl/gPYQHq
- Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, et al. (2008) Proliferation-promoting effect of platelet-rich plasma on human adipose derived stem cells and human dermal fibroblasts. Plast Reconstr Surg 122: 1352-1360. Link: https://goo.gl/azoiHj
- Gimble J, Guilak F (2003) Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5: 362-369. Link: https://goo.gl/m1GK1M
- Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F (2003) Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res 412: 196-212. Link: https://goo.gl/wKw2w0
- Tholpady SS, Llull R, Ogle RC, Rubin JP, Futrell JW, et al. (2006) Adipose tissue: stem cells and beyond. Clin Plast Surg 33: 55-62. Link: https://goo.gl/fyldAQ
- Stosich MS, Bastian B, Marion NW, Clark PA, Reilly G, et al. (2007) Vascularized adipose tissue grafts from human mesenchymal stem cells with bioactive cues and microchannel conduits. Tissue Eng 13: 2881-2890. Link: https://goo.gl/ryoHHd
- Otto TC, Lane MD (2005) Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol 40: 229–242. Link: https://goo.gl/cJflnD
- von Heimburg D, Zachariah S, Heschel I, Kühling H, Schoof H, et al. (2001) Human preadipocytes seeded on freeze-dried collagen scaffolds investigated In vitro and in vivo. Biomaterials 22: 429-438. Link: https://goo.gl/GTNx4H
- Sinha MK, Opentanova I, Ohannesian JP, Kolaczynski JW, Heiman ML, et al. (1996) Evidence of free and bound leptin in human circulation.Studies in lean and obese subjects and during short-term fasting.J Clin Invest98: 1277–1282. Link: https://goo.gl/itpME6
- Rodeheffer MS, Horowitz MC (2016) Fat Decisions: Leptin Regulates Bone versus Fat in the Marrow. Cell Stem Cell 18: 684-686. Link: https://goo.gl/QT6NtY
- Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ (2016) Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell 18: 782-796. Link: https://goo.gl/NOvfAw
- Chen T, Wu YW, Lu H, Guo Y, Tang ZH (2015) Adiponectin enhances osteogenic differentiation in human adipose-derived stem cells by activating the APPL1-AMPK signaling pathway. Biochem Biophys Res Commun 461: 237-242. Link: https://goo.gl/M8Iapq
- Yu L, Tu Q, Han Q, Zhang L, Sui L, et al. (2015) Adiponectin regulates bone marrow mesenchymal stem cell niche through a unique signal transduction pathway: an approach for treating bone disease in diabetes. Stem Cells 33: 240-252. Link: https://goo.gl/EECQG2
- Qiao L, Lee B, Kinney B, Yoo HS, Shao J (2011) Energy intake and adiponectin gene expression. Am J Physiol Endocrinol Metab 300: E809–E816. Link: https://goo.gl/nimWHu
- Lian J, Stewart C, Puchacz E, Mackowiak S, Shalhoub V, et al. (1989) Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci U S A 86: 1143–1147. Link: https://goo.gl/NlWKT4
- Lian J B, Stein GS (2003) The temporal and spatial subnuclear organization of skeletal gene regulatory machinery: Integrating multiple levels of transcriptional control. Calcif Tissue Int. 72: 631-637. Link: https://goo.gl/ZcO0U0
- Ryoo HM, Hoffmann HM, Beumer T, Frenkel B, Towler DA, et al. (1997) Stage-specific expression of Dlx-5 during osteoblast differentiation: involvement in regulation of osteocalcin gene expression. Mol Endocrinol 11:1681–1694. Link: https://goo.gl/G2QxbE
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-228. Link: https://goo.gl/9JqmEI
- McLimans W F, Giardinello F E, Davis EV, Kuecera CJ, Rake GW (1957) Submerged culture of mammalian cells: 5 L fermentor. J Bacteriol 74: 768-774. Link: https://goo.gl/u36y3K
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP (1997) Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem 64: 295-312. Link: https://goo.gl/gGZHnk
- Peng H, Zhang X, Du ZW , Zhang JW (2007) Expression of human HZF1 in E. coli and preparation of antibody against human HZF1 protein. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 29: 772 - 776. Link: https://goo.gl/TVhi4W
- Rubin C, Esteban E, Kieszak S, Hill RH, Dunlop B, et al. (2002) Assessment of human exposure and human health effects after indoor application of methyl parathion in Lorain County, Ohio, 1995-1996. Environ Health Perspect 110: 1047-1051. Link: https://goo.gl/C4qApH
- Undale AH , Westendorf JJ , Yaszemski MJ , Khosla S (2009) Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin Proc 84: 893-902. Link: https://goo.gl/exqe0w
- Ryu YJ, Cho TJ, Lee DS, Choi JY, Cho J (2013) Phenotypic characterization and in vivo localization of human adipose-derived mesenchymal stem cells. Mol Cells. 35: 557-564. Link: https://goo.gl/uOy7ky
- Csaki C, Matis U, Mobasheri A, Ye H, Shakibaei M (2007) Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study. Histochem Cell Biol 128: 507-520. Link: https://goo.gl/squFy1
- Pascucci L, Mercati F, Marini C, Ceccarelli P, Dall'Aglio C, et al. (2010) Ultrastructural morphology of equine adipose-derived mesenchymal stem cells. Histol Histopathol 25: 1277-1285. Link: https://goo.gl/KljRFV
- Liao Y, Zeng Z, Lu F, Dong Z, Chang Q, et al. (2015) In vivo dedifferentiation of adult adipose cells. PLoS ONE 10: e0125254. Link: https://goo.gl/q4k0iu
- Masamoto Y, Arai S, Sato T, Takamoto I, Kubota N, et al. (2015) Adipocyte-derived adiponectin positively regulates exit from quiescence of hematopoietic stem cells by potentiating mTORC1 activation after myelotoxic injury. Blood 2015: 126:777. Link: https://goo.gl/2FMMKd
- Li W, Xu L, Chen Y, Mu L, Cheng M, et al.(2016) Effect of estrodiol on leptin receptors expression in regulating fat distribution and adipocyte genesis. Gynecol Endocrinol 32(6):464-468. Link: https://goo.gl/Q45slM
- Shen L, Glowacki J, Zhou S (2011) Inhibition of adipocytogenesis by canonical WNT signaling in human mesenchymal stem cells. Exp Cell Res 317: 1796-803. Link: https://goo.gl/lUuZNE
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, et al. (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792. Link: https://goo.gl/G1PUW9
- Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A (2008) Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci 114: 361–374. Link: https://goo.gl/uMkEMt
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, et al. (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79 – 83. Link: https://goo.gl/BmgFp4
- Hawang CS, Loftus TM, Mandrup S, Lane MD (1997) Adipocyte differentiation and leptin expression. Annu Rev Cell Dev Biol13: 231- 259. Link: https://goo.gl/f3Mb43
- Wrann CD, Eguchi J, Bozec A, Xu Z, Mikkelsen T, et al. (2012) FOSL2 promotes leptin gene expression in human and mouse adipocytes J Clin Invest 1223: 1010-1021. Link: https://goo.gl/KIUHsW
- Lee JY, Hashizaki H, Goto T, Sakamoto T, Takahashi N, et al.(2011) Activation of peroxisome proliferator-activated receptor-α enhances fatty acid oxidation in human adipocytes. BiochemBiophys Res Commun 407: 818-22. Link: https://goo.gl/7fXier
- Deng T, Sieglaff DH, Zhang A, Lyon CJ, Ayers SD, et al.(2011) A peroxisome proliferator-activated receptor gamma (PPARgamma) /PPAR gammacoactivator 1beta autoregulatory loop in adipocyte mitochondrial function. J Biol Chem 286: 30723-30731. Link: https://goo.gl/CrWMF5
- Gonzales AM, Orlando RA (2007) Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr Metab 30: 4-22. Link: https://goo.gl/CugU8c
- Hietanen E, Greenwood MRC (1977) A comparison of lipoprotein lipase activity and adipocyte differentiation in growing male rats. J Lipid Re18: 480-490. Link: https://goo.gl/umr2ar
- Brown JK, Idris AM, Alteri C, Stenger DC (2002) Emergence of a New Cucurbit-Infecting Begomovirus Species Capable of Forming Viable Reassortants with Related Viruses in the Squash leaf curl virus Cluster. Phytopathology 92: 734-742. Link: https://goo.gl/XnSNv1
- Spiegelman BM, Puigserver P, Wu Z. (2000) Regulation of adipogenesis and energy balance by PPARgamma and PGC-1. Int J Obes Relat Metab Disorder 24: 8-10. Link: https://goo.gl/U3Evtz
- Brown CF, Yan J, Han TT, Marecak DM, Amsden BG, Flynn LE (2015) Effect of decellularized adipose tissue particle size and cell density on adipose-derived stem cell proliferation and adipogenic differentiation in composite methacrylated chondroitin sulphate hydrogels. Biomed Mater 10: 045010. Link: https://goo.gl/6PQvNj
- Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, et al. (2002) Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. IJ Orthop Res 20: 1060–1069. Link: https://goo.gl/nbrvTo
- zurNieden NI, Kempka G, Ahr HJ (2003) In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation 71: 18-27. Link: https://goo.gl/M4iizt
- Calabrese G, Giuffrida R, Fabbi C, Figallo E, Lo Furno D, et al.(2016) Collagen-Hydroxyapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In vitro. Vanella L, ed. PLoS ONE 11: e0151181. Link: https://goo.gl/1JWklK
- Benoit DS, Durney AR, Anseth KS (2006) Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng 12:1663–1673. Link: https://goo.gl/8psA0V
- El-Amin SF, Botchwey E, Tuli R, Kofron MD , Mesfin A , et al. (2016) Human osteoblast cells: isolation, characterization, and growth on polymers for musculoskeletal tissue engineering. J Biomed Mater Res A 76: 439–449. Link: https://goo.gl/jXTgOA
- Chen B, Lin H, Zhao Y, Wang B, Zhao Y, et al. (2007) Activation of demineralized bone matrix by genetically engineered human bone morphogenetic protein-2 with a collagen binding domain derived from von Willebrand factor propolypeptide. J Biomed Mater Res A 2007; 80: 428–434. Link: https://goo.gl/Lyppfo
- Tseng LF, Wang J, Baker RM, Wang G, Mather PT, et al. (2016) Osteogenic Capacity of Human Adipose-DerivedStem Cells is Preserved Following Triggering of Shape Memory Scaffolds. Tissue Eng Part A. TEA.2016.0095. Link: https://goo.gl/x8EPUK
- Peister A, Woodruff MA, Prince JJ, Gray DP, Hutmacher DW, et al. (2011) Cell sourcing for bone tissue engineering: Amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res 7: 17-27. Link: https://goo.gl/ft9Ehu
- Landim de Barros T, Brito VG, do Amaral CC, Chaves-Neto AH, Campanelli AP, et al. (2016) Osteogenic markers are reduced in bone-marrow mesenchymal cells and femoral bone of young spontaneously hypertensive rats. Life Sci 146: 174-183. Link: https://goo.gl/uump4W
- Silva W.A., Covas D., Panepucci R.A., Proto-Siqueira R., Siufi J., Zanette D., Santos A., Zago MA (2003) The Profile of Gene Expression of Human Marrow Mesenchymal stem cells. Stem Cells, 21: 661-669. Link: https://goo.gl/bmhggg
- Mygind T, Stiehler M, Baatrup A, Li H, Zou X, et al. (2007) Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials; 28: 1036-1047. Link: https://goo.gl/oWVZL3
- Viti F, Landini M, Mezzelani A, Petecchia L, Milanesi L, et al.(2016) Osteogenic Differentiation of MSC through Calcium Signaling Activation: Transcriptomics and Functional Analysis. Gronthos S, ed. PLoS ONE 11: e0148173. Link: https://goo.gl/fHKbOJ
- Tsupykov O, Ustymenko A, Kyryk V, Smozhanik E, Yatsenko K, et al. (2016) Ultrastructural study of mouse adipose-derived stromal cells induced towards osteogenicdirection. Microsc Res Tech 79: 557-564. Link: https://goo.gl/PWJCnN
- Chen FH, Tuan RS (2008) Adult stem cells for cartilage tissue engineering and regeneration. Curr Rheumatol Rev 4: 161-170. Link: https://goo.gl/kMycuI
- Åberg T, Rice R, Rice D, Thesleff I ,Waltimo-Sirén J (2005) Chondrogenic potential of mouse calvarial mesenchyme. J. Histoch Cytoch 53: 653–663. Link: https://goo.gl/7Ne71N
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
