Studies on Stem Cells Research and Therapy
Human Umbilical Cord Mesenchymal Stem Cells Suppress Systemic Lupus Erythematosus Lesions by Rebalancing CD4+/CD8+ Cell Population
Chan Li1#, Hongfang Ju2#, Qiongshu Li1#, Xin Zhou1, Xiaoping Zeng1, Yixin He1, Guifang Zeng1, Jia Liu1, Chunfeng Wu1, Tenglong Yan1, Wei Li3, Jiuwei Cui3, Xiang Hu1*, Ji-Fan Hu3,4* and Tao Li1*
2Obstetrics Department, Taizhou People’s Hospital, Taizhou, Jiangsu
3Stem Cell and Cancer Center, First Hospital, Jilin University, Changchun, Jilin, P. R. China
4Stanford University Medical School, Palo Alto Veterans Institute for Research, Palo Alto, CA, USA
#Equal contribution to the project
Xiang Hu, Shenzhen Beike Cell Engineering Research Institute, Shenzhen, Guangdong, P. R. China, Tel: 86 755 86309298, Fax: 86 755 86309309, E-mail: [email protected]
Tao Li, Shenzhen Beike Cell Engineering Research Institute, Shenzhen, Guangdong, P. R. China Tel.: 86 755 86026162; Fax: 86 755 86026165, E-mail: [email protected]
Cite this as
Li C, Ju H, Zhou X, Zeng X, He Y, et al. (2015) Human Umbilical Cord Mesenchymal Stem Cells Suppress Systemic Lupus Erythematosus Lesions by Rebalancing CD4+/CD8+ Cell Population. Studies on Stem Cells Research and Therapy 1(1): 004-011. DOI: 10.17352/sscrt.000003Despite considerable advances in the treatment for systemic lupus erythematosus (SLE), there is still an unmet need to develop novel therapeutic approaches with improved efficacy and lower side effects. Here we explore human umbilical cord-derived mesenchymal stem cells (hUCMSCs) as a promising treatment for SLE induced by concanavalin A-activated spleno-lymphocyte in BALB/c mice. The isolated hUCMSCs, carrying specific MSC cell surface markers (CD105, CD73 and CD90), exhibited the potential to differentiate into osteogenic and adipogenic lineages. In mice with SLE, transplantation of hUCMSCs improved disease symptoms by decreasing the levels of serum autoantibody (anti-dsDNA and anti-nuclear) and cytokines (TNF-α and IFN-γ). The cell therapy significantly alleviated renal lesions by lowering serum urea nitrogen, creatine and uric acid, and increasing albumin. Using immunohistochemical staining, we found that that hUCMSCs decreased endocapillary hypercellularity, glomerular degeneration, and complement C3 immune complex deposition in the kidney. Mechanically, the therapy with hUCMSCs decreased CD4+/CD8+ cell ratio in animals. These data suggest that hUCMSCs may modulate autoimmunity in SLE mice by rebalancing CD4+/CD8+ cell population. Transplantation of hUCMSCs may be explored as a promising alternative approach in the treatment of human lupus.
Introduction
Systemic lupus erythematosus (SLE), a chronic autoimmune disease with activation and proliferation of auto reactive T cells and B cells, is characterized by the production of autoantibody profile, such as anti-double stranded DNA (ds-DNA), anti-single stranded DNA (ss-DNA), and anti-nuclear antibody (anti-ANA), and the release of cytokines, including tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) [1-3]. In both human SLE and murine lupus models, such as MRL/lpr and NZB/W (F1), the kidney is the major site of injury and immune complex formation and/or deposition [4]. The conventional treatment of SLE mainly relies on high doses of cyclophosphamide (CYC), glucocorticoids, mycophenolate mofetil (MMF), and other immunosuppressive and biological agents. Although these recipes have markedly improved the outcome in SLE [5-7], disease control remains unsatisfactory and severe side effects were induced in a subset of patients [8-12]. Thus, there is still an unmet need to develop more effective, but less toxic treatments.
In the last decade, hematopoietic stem cell transplantation (HSCT) has been reported as a promising therapy to achieve treatment-free, long-term remission in lupus [13], but the rates of relapse and treatment related toxicity are high, as are the rates of the development of a secondary autoimmune disorder [14]. On the other hand, mesenchymal stem cells (MSCs) are widely studied as an alternative cell source for their ability to differentiate into multiple mesenchymal lineages, including adipocytes, osteoblasts, chondrocytes, myocytes and tenocytes [15-17]. An important function of MSCs for autoimmune diseases is their broad immunomodulatory effect on various activated lymphoid cells, such as T cells, B cells, natural killer cells, and dendritic cells [18-20]. Therapeutic MSCs can be isolated from various tissues, including placenta, bone marrow, amniotic fluid, muscle, tooth, adipose tissue and umbilical cord blood.
Recently, studies have focused on the use of human umbilical cord-derived MSCs (hUCMSCs). As an alternative to MSCs, hUCMSCs possess clear advantages, including easy production from a readily available source, low immunogenic potency, and high proliferative activity [21,22]. Several studies have reported the therapeutic effect of hUCMSCs in bone regeneration, liver cirrhosis and acute liver failure models [23-25]. In addition, hUCMSCs have been shown to have immunosuppressive properties in reducing inflammation in some autoimmune disease [26-28]. Therapeutic and preventive effects of hUCMSCs in the context of SLE, however, have not yet been well explored. In this study, we isolated MSCs from human umbilical cord and determined their potential therapeutic effect in a SLE mouse model induced by concanavalin A (ConA)-activated spleno-lymphocyte.
Materials and Methods
Induction of experimental SLE in BALB/c mice
In this study, we induced SLE in BALB/c mice following the method as previously described [29]. BALB/c mice were chosen to establish the SLE model due to their high response to the immunogen [4,30]. Female BALB/c mice, aged 5-6 weeks, were purchased from the Guangdong Laboratory Animal Center (Guangdong, China). Animals were maintained in specific pathogen-free conditions and environment under controlled conditions of light and humidity. Animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory following the protocol approved by Institutional Animal Ethic Committee of Shenzhen Beike Cell Engineering Research Institute.
To generate the SLE model, spleno-lymphocytes were isolated from BALB/c mice and cultured in RPMI-1640 medium (Invitrogene, CA) containing 10% fetal calf serum (Invitrogene, CA), 100 µg/ml streptomycin, and 100 µg/ml penicillin. Aliquots of spleno-lymphocytes were stimulated by ConA (5.0 µg/ml), while others were kept in a mitogen-free medium for 72 h as non-activated control spleen cells. Spleno-lymphocyte proliferation was determined using a WST-1 cell viability assay according to the manufacturer’s protocol (Roche).
A total of 55 mice were subcutaneously immunized with 1 × 107 ConA-activated spleno-lymphocyte cells, three times at one‑week intervals to establish the SLE model. As the control, 15 mice were subcutaneously injected with 0.9% saline. To confirm whether SLE model was established successfully in mice, 5 SLE mice and 5 control mice were sacrificed and enzyme-linked immunosorbent assays (ELISA) were used to detect serum anti-dsDNA antibody.
Isolation and characterization of hUCMSCs
Umbilical cords were collected after normal deliveries as quickly as possible in hospital after obtaining written parenteral consent. Isolation of hUCMSCs was performed as described previously [25,31]. Briefly, Wharton’s jelly was isolated and cut into 1-mm2 pieces, treated with collagenase type 1 (Sigma, MO), and then were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum and antibiotics at 37℃ in a humidified atmosphere consisting of 5% CO2. Non-adherent cells were removed by washing and the cells that migrated out from the explants after 5-7 days were trypsinized and expanded for the study. The protocol was approved by the Human Medical Ethical Review Committee from Shenzhen Beike Cell Engineering Research Institute.
The hUCMSCs at passages 2-5 were used for transplantaton following rigorous quality control for cell viability, pathogenic microorganisms, hepatitis B surface antigen, hepatitis B core antibody, and hepatitis C virus antibody, human immunodeficiency virus antibodies I and II, cytomegalovirus IgM, and syphilis antibody. The immunophenotype of the culture-expanded hUCMSCs at passage 2 was analyzed by flow cytometry for specific cell surface markers (CD90, CD73 and CD105), hematopoietic cell markers (CD45, CD34, CD14 and CD19) and extracellular matrix receptors (CD29 and CD44) and major histocompatibility elements (HLA-DR) (all purchased from BD Biosciences, CA). Flow cytometry was performed with the use of FACSCalibur (BD Biosciences, CA).
Adipogenic and osteogenic differentiation
To validate the multipotency of the isolated hUCMSCs, differentiation to adipogenic and osteogenic lineages were performed as described previously [25,32]. After 3 weeks of induction, cells were stained with the oil red O or alizarin red to detect the presence of neutral lipid vacuoles in differentiated adipocytes or calcium deposition in osteocytes, respectively.
hUCMSCs transplantation
For treatment, 50 SLE mice were randomly divided into 5 groups: M1 (n = 10), M2 (n = 10), M3 (n = 10), M4 (n = 10), and positive control (PC) group (n = 10). They were transplanted with 3×105, 1×105, 3×104, 1×104 and 0 hUCMSCs per 10 g body weight, respectively. Another 10 mice treated with saline were used as a negative control (NC) and were not transplanted with hUCMSCs.
Serum parameter analyses
Blood samples of animals in all groups were collected through orbital bleeds. ELISA was carried out using commercial available kits (ADI, USA) to detect anti dsDNA antibodies, anti-ANA, and inflammatory cytokines, including TNF-α and IFN-γ. The serum levels of urea nitrogen (UREA), creatine (CREA), uric acid (UA) and albumin (ALB) were analyzed with an automated biochemical analyzer (Mindray, Shenzhen, China).
Lymphocyte subtypes assay
Spleno-lymphocytes (1 × 106 cells) isolated from animals in all groups were stained with FITC-anti-CD3 antibody, APC-anti-CD4 antibody and PE-anti-CD8 antibody and then analyzed on a FACS Calibur flow cytometer.
Assessment of renal pathology
Kidneys from treatment animals were dissected, fixed in 10% neutral-buffered formalin, dehydrated in ascending grades of ethyl alcohol, cleared in xylene and mounted in molten paraplst. Histological sections (5 µm) were cut and stained with Hematoxylin and Eosin (H&E), and Masson trichrome [33].
For immunohistochemical analyses, rabbit anti-mouse complement C3 antibody (ab11887, Abcam) and an Ultra SensitiveTM SP (Mouse/Rabbit) IHC Kit (KIT-9720, MaiXin. BIO, Fuzhou, China) were used to examine the presence of complement C3 according to the manufacturer’s protocol.
Statistical analysis
Data were expressed as the means ± SD. Statistical analysis was performed by SPSS 17.0 software. The Student’s t-test was used to compare serum parameters. P < 0.05 was considered to indicate a statistically significant result.
Results
Establishment of experimental SLE in BALB/c mice
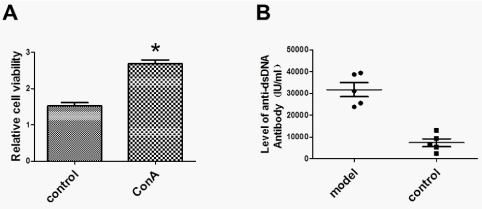
To induce SLE in BALB/c mice, spleno-lymphocytes from BALB/c mice were isolated, cultured and stimulated with ConA for 72 h and cell proliferation was detected by WST-1 assay. As shown in Figure 1A, cell proliferation was significantly increased by induction with 5 µg/ml ConA, indicating that spleno-lymphocytes were highly activated.
We used these activated spleno-lymphocytes to immunize BALB/c mice three times at one‑week intervals and performed ELISA assay to confirm the SLE model by detecting serum anti-ds DNA antibody, a typical character of SLE. As shown in Figure 1B, BALB/c mice immunized with activated spleno-lymphocytes had significantly high level of anti-ds DNA autoantibody in the serum compared to the control, indicating the successful generation of the SLE model in BALB/c mice.
Multipotency of hUCMSCs
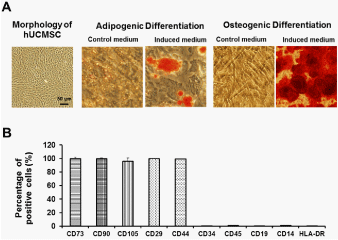
The immunophenotype of the culture-expanded hUMSCs at passage 4 was analyzed by flow cytometry for specific cell surface markers. As shown in Figure 2B, hUMSCs were positive for known MSC markers (CD105, CD73 and CD90) and were negative for hematopoietic markers (CD45, CD34, CD14, CD19 and HLA-DR). Two cell surface markers that have affinity for extracellular matrix elements, CD29 and CD44, were also expressed by isolated hUCMSCs.
To verify the lineage potential, hUMSCs were placed in six-well plates for adipogenic and osteogenic differentiation after 21 days of culture. Intracytoplasmic lipid droplets stained with oil red O and calcium deposits stained with alizarin red were observed (Figure 2A), demonstrating the potential of adipogenic and osteogenic differentiation of the isolated hUCMSCs. We thus used these multipotent hUCMSCs for transplantation studies.
Alleviation of SLE symptoms by hUCMSCs therapy
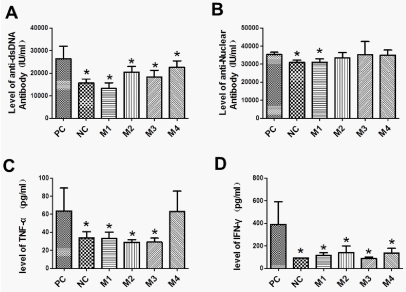
The therapeutic effect of hUCMSCs on SLE was examined by ELISA detection of anti-dsDNA antibody, anti-ANA antobody, TNF-α and IFN-γ. As shown in Figure 3, the levels of anti-dsDNA antibody, anti-ANA antibody, TNF-α and IFN-γ in SLE mouse model were highly induced in positive controls (the PC group) as compared with the negative control (the NC group). However, in treatment groups (M1, M2 and M3), hUCMSCs effectively decreased the levels of serum anti-dsDNA antibody, TNF-α and IFN-γ in SLE mice (p < 0.05). The level of serum ANA in M1 group was also slightly reduced. These results indicate that hUCMSC transplantation has a therapeutic effect on SLE in BALB/c mice.
hUCMSCs reduce kidney injury in SLE mice
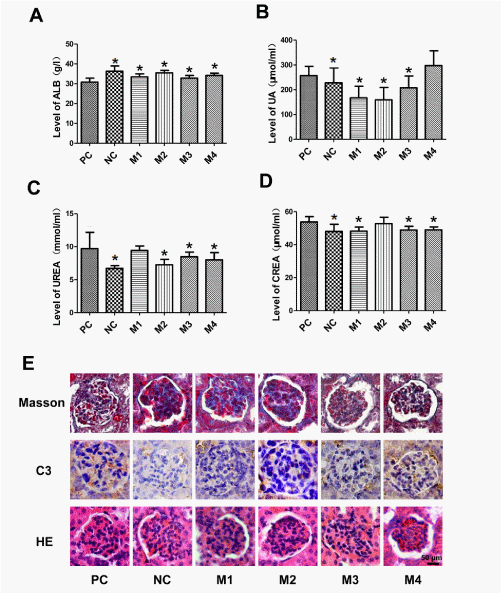
Kidney is a major site of damage and immune complex formation and/or deposition in SLE [4]. To further determine the effect of hUCMSCs on SLE, renal function and autoimmune injury were compared by serum biochemical assays to detect the levels of albumin (ALB), uric acid (UA), urea nitrogen (UREA) and creatine (CREA). As compared with the normal group (NC), the SLE mice (PC) exhibited the reduction in renal ALB and the increment in renal UA, UREA and CREA. We found that hUCMSC transplantation decreased the levels of serum UA, UREA and CREA, but increased the level of ALB to the level as seen in normal mice (NC) (Figures 4A-4D). Assessment of renal pathology and immunohistochemical staining showed that hUCMSCs decreased endocapillary hypercellularity, glomerular degeneration and complement C3 immune complex deposition in kidneys of SLE model mice (Figure 4E). These results suggest that treatment of hUCMSCs alleviates kidney injury in the SLE BALB/c mouse model.
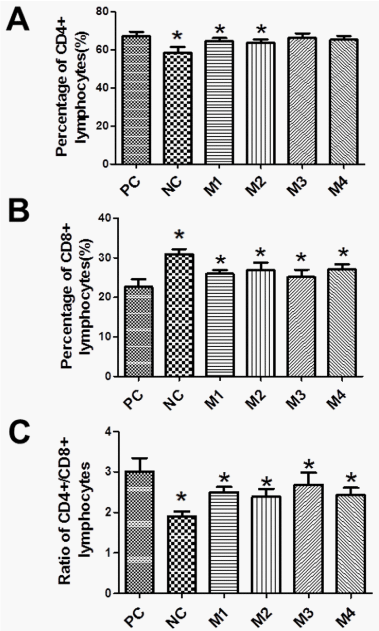
hUCMSCs transplantation rebalances lymphocyte subtypes
T-lymphocytes play a pivotal role in the pathogenesis of SLE [34]. hUCMSCs have been reported to regulate lymphocytes proliferation through paracrine mechanisms, thus playing a safe and effective treatment of some autoimmune diseases. To verify whether hUCMSCs have effect on lymphocyte subtypes, we analyzed CD4+ and CD8+ T cell subtypes after gated on CD3+ T cell.
As shown in Figure 5, the percentage of CD3+ CD4+ T cell was significantly higher in the PC group than that in the NC control. High doses of hUCMSCs transplantation, especially in group M2, decreased CD3+ CD4+ cells (Figure 5A). On the other hand, the CD3+ CD8+ T cell population was lower in the PC group than that in the NC group. Treatment with hUCMSCs in M1, M2 and M3 groups increased the CD3+ CD8+ cell population (Figure 5B). Taken together, hUCMSC transplantation decreased the CD4+/CD8+ ratio in SLE BALB/c mice (Figure 5C). These results suggest that hUCMSCs may modulate autoimmunity by shifting the CD4+/CD8+ lymphocyte subtypes in SLE mice.
Discussion
SLE is a severe and thorny disease that often represents a therapeutic challenge because of its heterogeneous organ manifestations [1,2,35]. hUCMSCs have shown marked therapeutic effects in a number of animal disease studies, and may serve as a promising therapeutic tool for the treatment of patients with severe and refractory autoimmune diseases [5,10,11]. However, the therapeutic effects of hUCMSCs in the context of SLE have not yet been well explored. In the present study, we successfully established experimental SLE in BALB/c mice using ConA-activated spleno-lymphocyte to investigate the therapeutic potential of hUCMSCs.
SLE can occur spontaneously in certain strains of mice, such as NZB/W (F1) and MRL/pr with multiple genetic loci that accelerate the onset of the disease [4,30]. Lupus also can be induced in BALB/c mice by intraperitoneal injection of pristane, a chemical component, or special antigen in non-autoimmune prone strains [4,30]. It has been reported that concanavalin A-activated spleno-lymphocytes could induce SLE-like syndrome in BALB/c mice, a strain usually not considered to be genetically susceptible to lupus [29]. All these murine lupus models facilitate studies on the relationship between autoantibody formation and organ damage, and help the screening for effective treatment. In this study, we chose ConA-activated spleno-lymphocytes to induce SLE mouse model in BALB/c mice [29]. This model was efficient and rapid. We isolated spleno-lymphocytes from BALB/c mice and stimulated them with ConA. We found that spleno-lymphocytes were highly activated after treated with ConA. ELISA and kidney pathogenicity assays showed that the syndrome induced by splenophocytes shares many of the characteristics of human lupus, including both clinical features (glomerular hypercellularity, glomerular Ig deposition, proteinuria) and the autoantibody profile (anti ds-DNA, ANA), with complex immune abnormality [35].
To determine the effect of hUCMSCs on SLE mouse model, hUCMSCs were isolated, cultured and evaluated in vitro for osteogenic and adipogenic differentiation potential and immunophenotype. The results showed that hUCMSCs can be differentiated into osteogenic and adipogenic lineages and possess specific MSC cell surface markers (CD105, CD73 and CD90). Thus, we investigated the therapeutical potential in the SLE mouse model. SLE is characterized by the production of autoantibodies to nuclear proteins and nucleic acids, accompanied with clinical manifestation (such as leukopenia and thrombocytopenia) and kidney damage [1,3,4]. Al-Mutairi et al reported that proinflammatory cytokines (TNF-α, IFN-γ, IL-8, IL-6) were more prevalent in the serum of SLE patients [36]. Our data indicated that high-dose hUCMSC transplantation reduced levels of serum autoantibodies (anti ds-DNA, ANA) and inflammatory cytokines (TNF-α, IFN-γ), and improved renal function in SLE mouse model, suggesting that hUCMSCs has a therapeutical effect on SLE established in BALB/c mice.
In this study, we treated the SLE animals with four doses of hUCMSCs (3×105, 1×105, 3×104, and 1×104 per 10 g body weight). However, the therapeutic effect of hUCMSCs on SLE-induced mice was not in a typical dose-dependent manner. This is especially true for the renal parameters. For example, the level of urea nitrogen was elevated in the SLE mice (the PC group) as compared with the normal control (the NC group) (Figure 4C). We observed a significant improvement in renal urea nitrogen in the M2-M4 groups but not in the high hUCMSC group (the M1 group). We believe that the lack of dose-dependent effect may be related to the big variation between treatment groups. Future studies are needed to conform the therapeutic role of hUCMSCs in a more sensitive animal model.
Naturally arising regulatory T cells (Treg) play a pivotal role in the pathogenesis of SLE. The best characterized Treg cells, a subset of CD4+ T-helper cells expressing high levels of the interleukin-2 receptor α- chain CD25 and the transcription factor forkhead box P3 (FoxP3) [37], have the ability to suppress CD8+ T cells and NK cells, to inhibit aberrant immune responses, to regulate peripheral T-cell homeostasis, and to maintain self-tolerance by secreting immunoregulation factors such as IL-4, IL-10 and TGF-β [38-40]. Unbalanced CD4+ cell population, like decreased CD4+CD25+ or increased CD4+CD25- can lead to the disorder of Th1/Th 2 and CD4+/CD8+ ratio, resulting in hyperfunction of humoral immunity, as in the case of SLE [41-43]. Recently, CD8+T-cell populations have also been shown to possess immunosuppressive function, including CD8+CD28-, CD8+IL-10+ and CD8+CD25+ T cells, which can inhibit the activation and proliferation of lymphocytes [44]. CD8+CD28- T cells have attracted interest for their critical roles in various chronic immune disorders, such as Graves’s disease, rheumatoid arthritis and type 1 diabetes mellitus [45,46]. Tulunay et al. [47], showed that CD8+CD28- population was lower in patients with SLE than in healthy individuals. These results indicate that both CD4+ and CD8+ T cells play an important role in SLE.
The specific mechanism underlying the therapeutic role of hUCMSCs in SLE is still unclear. Accumulating evidence have demonstrated that MSCs have immune modulatory properties, these cells have attracted significant attention for their potential to treat immune related diseases, such as autoimmune diseases, graft-versus-host disease (GVHD), liver diseases, and tissue inflammation [48, 49]. MSCs have been reported to suppress T cell responses, including proliferation, activation and inflammatory cytokine production. Some studies have suggested that MSCs also inhibit the cytotoxic activity of natural killer cells, regulate the maturation and function of B cells and help determine the polarization of macrophages. Studies have shown that hUCMSCs could recruit, modulate and maintain the function and phenotype of Treg cells over time [50]. Mechanistically, MSCs-derived TGF-β and PGE2 are key regulators involved in Treg cell induction [51,52]. In the present study, we analyzed the CD4+ and CD8+ lymphocyte subtypes and found that hUCMSCs transplantation rebalanced lymphocyte subtypes in SLE mice by decreasing CD3+CD4+ cells and increasing CD3+CD8+ cells. It is clear that increased CD4+CD25- T-helper cells can trigger the disorder of Th1/Th2, resulting in SLE, while CD8+T-cell including CD8+CD28-, CD8+IL-10+ and CD8+CD25+ T cells possess immunosuppressive function [40-43]. Thus, our data suggest that hUCMSCs may achieve the desired therapeutic effect on SLE through a mechanism of rebalancing the autoimmune-related lymphocyte subtypes.
It should be noted that transplantation of MSCs from other sources have been shown to escalate Treg population as a mechanism of exerting immunosuppression [53,54]. For example, in a 25-year-old severe SLE patient with multiple life-threatening complications and refractory to conventional cyclophosphamide (CYC) therapy, Wang et al. [55], reported that transplantation of autologous hematopoietic stem cell and MSCs re-established self-tolerance in SLE by increasing CD4+CD25+FoxP3+Treg cells in PBMCs. In this case, CD4+CD25+ regulatory T cells (Treg) act to control the self-antigen–reactive T cells in autoimmune diseases. Similarly, MSC-induced tolerance is associated with the expansion of CD4(+)CD25(+)Foxp3(+) Treg cells in other two models [56,57]. Currently, we are not sure if similar mechanisms are also involved in our model. Thus, it would be interesting to assess the percentage of regulatory T cells in the spleen and peripheral blood in SLE mice treated with hUCMSCs.
Several studies have reported the ratio of CD4/CD8 T-cells in healthy Chinese, Malaysian, Indian and Caucasian populations, ranging from 0.6 to 2.8 [58]. In addition, the reference value of lymphocyte immune phenotype varies in race, age and environment [59]. Currently, there are no specific cut points for CD4/CD8 T-cell ratio in SLE. In our mouse study, we found that the ratio of CD4/CD8 T-cells was 3.004 in the PC group, 1.899 in the NC group, and 2.424 to 2.678 in the hUCMSCs-treated group, respectively. There was a clear therapeutic effect by hUCMSCs in the reduction of the CD4/CD8 T-cell ratio in mice with SLE. Future studies are required to address the specific role of the CD4/CD8 T-cell ratio in the pathogenesis of SLE.
Since SLE is also depicted as abnormal humoral immune disease, thus B cells also play a central role in the immunopathogenesis via antibody-dependent and antibody-independent ways. Excessive autoantibodies production, hyperresponsiveness and prolonged survival of auto reactive B cells are characteristics of SLE [60]. These B cells-related characteristics were not identified, which is a limitation in present research. We speculate that B cells would also involve in mechanisms of hUCMSCs- improved SLE.
In summary, our study has demonstrated that hUCMSCs exert a therapeutic effect in a SLE BALB/c mouse model. The therapeutical role of hUCMSCs may be related to its ability to modulating autoimmunity by rebalancing CD4+/CD8+ cell population in animals. Thus, hUCMSC therapy may have a potential application in the treatment of human lupus.
This study was supported by Shenzhen Research Grant (#JSGG20130917151830203) and (#KC2013JSJS0014A) X.H and T.L.; Shenyang Research Grant (#F13-205-9-00) to T.L.; California Institute of Regenerative Medicine (CIRM) grant (#RT2-01942), Jilin International Collaboration Grant (#20120720), and the National Natural Science Foundation of China grant (#81272294, #31430021) to J.F.H.; National natural science foundation of China (#81372835) and Jilin international collaboration grant (20130413010GH) to W.L.; the grant of Key Project of Chinese Ministry of Education (#311015) to C.J.
- Fernandez D, Perl A (2009) Metabolic control of T cell activation and death in SLE. Autoimmunity reviews 8: 184-189.
- Peng SL (2009) Altered T and B lymphocyte signaling pathways in lupus. Autoimmunity reviews 8:179-183.
- Rahman A, Isenberg DA (2008) Systemic lupus erythematosus. The New England journal of medicine 358:929-939.
- Brosh N, Eilat E, Zinger H, Mozes E (2000) Characterization and role in experimental systemic lupus erythematosus of T-cell lines specific to peptides based on complementarity-determining region-1 and complementarity-determining region-3 of a pathogenic anti-DNA monoclonal antibody. Immunology 99:257-265.
- Wang D, Zhang H, Liang J, Li X, Feng X, et al. (2013) Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell transplantation 22:2267-2277.
- Crow MK (2009) Developments in the clinical understanding of lupus. Arthritis research & therapy 11:245.
- Karim MY, Pisoni CN, Khamashta MA (2009) Update on immunotherapy for systemic lupus erythematosus--what's hot and what's not! Rheumatology (Oxford) 48:332-341.
- Houssiau FA, Ginzler EM (2008) Current treatment of lupus nephritis. Lupus 17:426-430.
- Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, et al. (2004) B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis and rheumatism 50:2580-2589.
- Katsifis GE, Tzioufas AG (2004) Ovarian failure in systemic lupus erythematosus patients treated with pulsed intravenous cyclophosphamide. Lupus 13:673-678.
- Navarro-Zarza JE, Alvarez-Hernandez E, Casasola-Vargas JC, Estrada-Castro E, Burgos-Vargas R (2010) Prevalence of community-acquired and nosocomial infections in hospitalized patients with systemic lupus erythematosus. Lupus 19:43-48.
- Ruiz-Irastorza G, Olivares N, Ruiz-Arruza I, Martinez-Berriotxoa A, Egurbide MV, et al. (2009) Predictors of major infections in systemic lupus erythematosus. Arthritis research & therapy 11:R109.
- Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, et al. (2009) Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood 113:214-223.
- Loh Y, Oyama Y, Statkute L, Quigley K, Yaung K, et al. (2007) Development of a secondary autoimmune disorder after hematopoietic stem cell transplantation for autoimmune diseases: role of conditioning regimen used. Blood 109:2643-2648 .
- Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71-74.
- Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739-2749.
- Noth U, Rackwitz L, Steinert AF, Tuan RS (2010) Cell delivery therapeutics for musculoskeletal regeneration. Advanced drug delivery reviews 62:765-783.
- Nauta AJ, Fibbe WE (2007) Immunomodulatory properties of mesenchymal stromal cells. Blood 110:3499-3506.
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, et al. (2005) Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105:4120-4126.
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M (2006) Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24:74-85.
- Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, et al. (2008) Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells 26:2865-2874.
- Cho PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, et al. (2008) Immunogenicity of umbilical cord tissue derived cells. Blood 111:430-438.
- Byeon YE, Ryu HH, Park SS, Koyama Y, Kikuchi M, et al. (2010) Paracrine effect of canine allogenic umbilical cord blood-derived mesenchymal stromal cells mixed with beta-tricalcium phosphate on bone regeneration in ectopic implantations. Cytotherapy 12:626-636.
- Wang J, Zhou X, Cui L, Yan L, Liang J, et al. (2010) The significance of CD14+ monocytes in peripheral blood stem cells for the treatment of rat liver cirrhosis. Cytotherapy 12:1022-1034.
- Liu Z, Meng F, Li C, Zhou X, Zeng X, et al. (2014) Human umbilical cord mesenchymal stromal cells rescue mice from acetaminophen-induced acute liver failure. Cytotherapy 16:1207-1219.
- Li D, Han Y, Zhuang Y, Fu J, Liu H, et al. (2015) Overexpression of COX-2 but not indoleamine 2,3-dioxygenase-1 enhances the immunosuppressive ability of human umbilical cord-derived mesenchymal stem cells. International journal of molecular medicine 35:1309-1316.
- Donders R, Vanheusden M, Bogie JF, Ravanidis S, Thewissen K, et al. (2014) Human Wharton?s jelly-derived stem cells display immunomodulatory properties and transiently improve rat experimental autoimmune encephalomyelitis. Cell transplantation. 24: 2077-98.
- Alunno A, Montanucci P, Bistoni O, Basta G, Caterbi S, et al. (2015) In vitro immunomodulatory effects of microencapsulated umbilical cord Wharton jelly-derived mesenchymal stem cells in primary Sjogren's syndrome. Rheumatology (Oxford) 54:163-168.
- Li H, Zhang YY, Sun YN, Huang XY, Jia YF, Li D (2004) Induction of systemic lupus erythematosus syndrome in BALB/c mice by immunization with active chromatin. Acta pharmacologica Sinica 25:807-811.
- Satoh M, Richards HB, Shaheen VM, Yoshida H, Shaw M, et al. (2000) Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clinical and experimental immunology 121:399-405.
- Lee WM, Stravitz RT, Larson AM (2012) Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology 55:965-967.
- Chen M, Zhang H, Wu J, Xu L, Xu D, et al. (2012) Promotion of the induction of cell pluripotency through metabolic remodeling by thyroid hormone triiodothyronine-activated PI3K/AKT signal pathway. Biomaterials 33:5514-5523.
- Du T, Zou X, Cheng J, Wu S, Zhong L, et al. (2013) Human Wharton's jelly-derived mesenchymal stromal cells reduce renal fibrosis through induction of native and foreign hepatocyte growth factor synthesis in injured tubular epithelial cells. Stem cell research & Therapy 4(3):59.
- Wang D, Feng X, Lu L, Konkel JE, Zhang H, et al. (2014) A CD8 T cell/indoleamine 2,3-dioxygenase axis is required for mesenchymal stem cell suppression of human systemic lupus erythematosus. Arthritis Rheumatol 66:2234-2245.
- Li H, Zhang YY, Huang XY, Sun YN, Jia YF, Li D (2005) Beneficial effect of tripterine on systemic lupus erythematosus induced by active chromatin in BALB/c mice. European journal of pharmacology 512:231-237.
- Al-Mutairi S, Al-Awadhi A, Raghupathy R, Al-Khawari H, Sada P, et al. (2007) Lupus patients with pulmonary involvement have a pro-inflammatory cytokines profile. Rheumatology international 27:621-630.
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155:1151-64.
- Maloy KJ, Powrie F (2001) Regulatory T cells in the control of immune pathology. Nature immunology 2:816-822.
- Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, et al. (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunological reviews 212:8-27.
- Sakaguchi S, Sakaguchi N (2005) Regulatory T cells in immunologic self-tolerance and autoimmune disease. International reviews of immunology 24:211-226.
- Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A (2004) CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol 112:258-267.
- Camara NO, Sebille F, Lechler RI (2003) Human CD4+CD25+ regulatory cells have marked and sustained effects on CD8+ T cell activation. European journal of immunology 33:3473-3783.
- Duthoit CT, Mekala DJ, Alli RS, Geiger TL (2005) Uncoupling of IL-2 signaling from cell cycle progression in naive CD4+ T cells by regulatory CD4+CD25+ T lymphocytes. J Immunol 174:155-163.
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY (2005) A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology 6:1142-451.
- Scarsi M, Ziglioli T, Airo P (2010) Decreased circulating CD28-negative T cells in patients with rheumatoid arthritis treated with abatacept are correlated with clinical response. J Rheumatol 37:911-916.
- Colovai AI, Mirza M, Vlad G, Wang S, Ho E, et al. (2003) Regulatory CD8+CD28- T cells in heart transplant recipients. Hum Immunol 64:31-37.
- Tulunay A, Yavuz S, Direskeneli H, Eksioglu-Demiralp E (2008) CD8+CD28-, suppressive T cells in systemic lupus erythematosus. Lupus 17:630-637.
- Jones BJ, Brooke G, Atkinson K, McTaggart SJ (2007) Immunosuppression by placental indoleamine 2,3-dioxygenase: a role for mesenchymal stem cells. Placenta 28:1174-1181.
- Li C, Zhang W, Jiang X, Mao N (2007) Human-placenta-derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cells. Cell Tissue Res 330:437-446.
- Liu Q, Zheng H, Chen X, Peng Y, Huang W, et al. (2014) Human mesenchymal stromal cells enhance the immunomodulatory function of CD8CD28 regulatory T cells. Cell Mol Immunol. 6: 708-18.
- Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A (2007) Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 92:881-888.
- English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, et al. (2009) Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 156:149-160.
- Li Y, Qu YH, Wu YF, Liu L, Lin XH, et al. (2015) Bone marrow mesenchymal stem cells suppressing activation of allogeneic cytokine-induced killer/natural killer cells either by direct or indirect interaction. Cell Biol Int 39:435-445.
- Stagg J, Galipeau J (2013) Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med 13:856-867.
- Wang Q, Qian S, Li J, Che N, Gu L, et al. (2015) Combined transplantation of autologous hematopoietic stem cells and allogenic mesenchymal stem cells increases T regulatory cells in systemic lupus erythematosus with refractory lupus nephritis and leukopenia. Lupus 24:1221-1226.
- Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, et al. (2008) Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 181:3933-3946.
- Zhang X, Ren X, Li G, Jiao C, Zhang L, et al. (2011) Mesenchymal stem cells ameliorate experimental autoimmune uveoretinitis by comprehensive modulation of systemic autoimmunity. Invest Ophthalmol Vis Sci 52:3143-3152.
- Reichert T, DeBruyère M, Deneys V, Tötterman T, Lydyard P, et al. (1991) Lymphocyte subset reference ranges in adult Caucasians. Clin Immunol Immunopathol 60(2):190-208.
- Choong ML, Ton SH, Cheong SK (1995) Influence of race, age and sex on the lymphocyte subsets in peripheral blood of healthy Malaysian adults. Ann Clin Biochem 32 ( Pt 6):532-539.
- Zhao L, Ye Y, Zhang X. (2015) B cells biology in systemic lupus erythematosus-from bench to bedside. Sci China Life Sci 58:1111-25.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
