Global Journal of Fertility and Research
Higher levels of Hepatocyte Growth Factor (HGF) in human seminal plasma in comparison with blood plasma and negative association with several motile sperm cells
Anders Larsson1*, Lena Carlsson1, Rasha Khierallah2, Jan Holte2 and Theodora Kunovac Kallak2
2Department of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden
Cite this as
Larsson A, Carlsson L, Khierallah R, Holte J, Kallak TK (2023) Higher levels of Hepatocyte Growth Factor (HGF) in human seminal plasma in comparison with blood plasma and negative association with several motile sperm cells. Glob J Fertil Res 8(1): 008-013. DOI: 10.17352/gjfr.000023Copyright
© 2023 Larsson A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Context: Semen is a complex fluid with many functions, some of them well-known, others more obscure.
Aims: The aim of this study was to investigate the levels of Hepatocyte Growth Factor (HGF) in human seminal plasma in comparison with blood plasma levels.
Methods: HGF concentrations were measured in seminal plasma from 40 men utilizing commercial ELISA kits. Blood plasma from 40 healthy blood donors served as a comparison group.
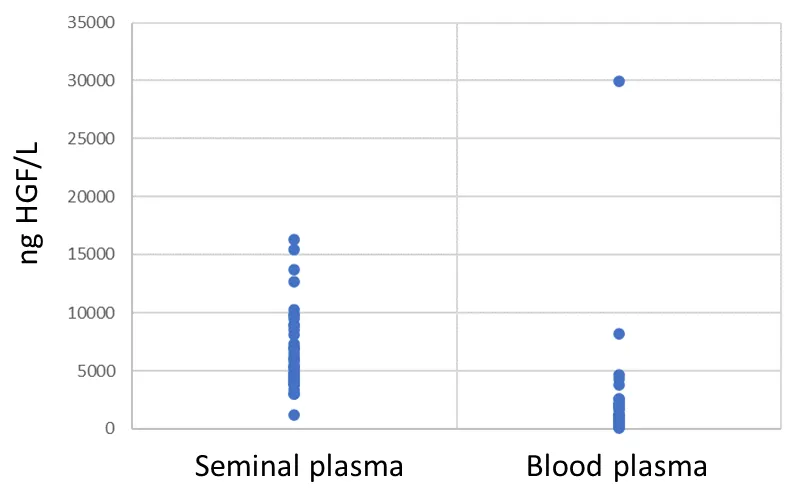
Results: Median seminal plasma HGF was approximately five times higher than the levels found in blood plasma (5717.5 pg/mL vs. 1124.6 pg/mL). There was a negative correlation between HGF values in seminal plasma and the number of sperm cells.
Conclusion: The study shows that seminal plasma contains high levels of HGF and that HGF binds to prostasomes. Male HGF can thus reach the female reproductive tract during unprotected sexual intercourse. Further studies are warranted to evaluate the effect of this on fertility.
Background
Assessment of male fertility is traditionally defined based on semen parameters such as sperm count, motility, morphology, pH, and volume [1]. Seminal fluid contains an array of cytokines, chemokines, and growth factors [2,3]. The most recent WHO guidelines propose that specific cytokines may be useful in the assessment of male infertility [4]. Seminal plasma interacts with the female genital tract and induces an inflammation-like response [5,6]. The local inflammatory response may also influence male fertility [7-9].
Hepatocyte Growth Factor (HGF), also called Scatter Factor (SF), is a cytokine that promotes paracrine cellular growth and motility [10]. It is mainly secreted by mesenchymal cells and acts on epithelial and endothelial cells. HGF belongs to the plasminogen subfamily of S1 peptidases [11]. HGF has been shown to have an important role in placental growth, post-implantation, and embryonic organ development [12,13].
Seminal plasma is composed of secretions produced mainly by the prostate gland, and the seminal vesicles. The remaining parts originate from the epididymis, testes, and Cowper’s glands [14,15].
Previous studies have shown the presence of HGF in seminal plasma and its receptor in testicular tissue and spermatozoa in men [16-18]. The highest HGF levels in seminal plasma were observed in azoospermic samples [19].
Immunohistochemical expression of HGF was significantly reduced in placentas of preeclampsia-associated pregnancies compared to uncomplicated ones [20]. Depletion of HGF in mice leads to placental maldevelopment and fetal lethality [21,22].
HGF has a number of important functions in the female reproductive tract. HGF promotes the growth and development of ovarian follicles and supports their maturation [23,24]. HGF stimulates the production of estrogen and progesterone. HGF is involved in the preparation of the uterus for embryo implantation [25]. During pregnancy, HGF is involved in the development and function of the placenta [26-28].
Seminal plasma has previously been shown to promote the growth of human epithelial and stroma cells from eutopic endometria obtained from women with endometriosis [29]. Pretreatment of the cells with a combination of anti-HGF and anti-prostaglandin E2 antibodies suppressed the growth of the endometrial cells. This indicates that HGF had an important role in the promotion of cell growth and that HGF present in the seminal fluid could have an impact on the female endometrium.
An increasing number of studies have demonstrated the importance of HGF and the HGF-receptor, a protein tyrosine kinase receptor, in the progression of prostate cancer [30,31] and the pathway has even been suggested as a therapeutic target for prostate cancer treatment [30].
Overall, HGF is essential for male fertility by supporting testicular development, promoting spermatogenesis and sperm maturation, contributing to seminal fluid production, and ensuring proper function of the male reproductive tract [32]. Dysregulation of HGF signaling can have implications for male reproductive health and fertility.
Considering the previously demonstrated roles of HGF in the female and male reproductive tract, the aim of the present study was to investigate the levels of HGF in human seminal plasma in comparison with human blood plasma and to test if the HGF could be present in the surface of prostasomes.
Materials and methods
Seminal plasma and blood plasma samples
A total of 40 patients attending the Carl von Linné Clinic, Uppsala, were included in the study. The samples were anonymized directly after sampling. The median age of the men was 34 years (range 25–66 years). The use of seminal fluid samples and blood plasma samples was approved by the ethical committee at Uppsala University (01-367). The ethical permit limited the patient information to age and sex and the use of surplus materials from clinical samples. Semen samples were collected by masturbation and after at least 3 days of abstinence. After liquefaction at room temperature (30–60 min), the samples were analyzed for the number of sperm cells and motile sperm cells as described earlier [33].
The total number of sperm cells was counted by manual microscopy of diluted sperm samples using a Bürker chamber. Two hundred sperm cells were then analyzed to calculate the number of motile sperm cells in relation to the total number of sperm cells [4]. After centrifugation at 1500 g for 20 min, the samples were initially frozen at -20 °C for and then transferred to -70 °C until tested.
A total of 40 EDTA plasma samples from healthy male blood donors were also analyzed to compare the HGF levels in seminal plasma and blood plasma.
Determination of hepatocyte growth factor
The analyses of HGF were performed without knowledge of clinical information using commercial sandwich ELISA kits (DY294, R&D Systems, Minneapolis, MN, USA), in which a monoclonal antibody specific for HGF was coated onto a microtiter plate. After blocking the plates with 40 mg/mL Bovine Serum Albumin (BSA), standards and samples were pipetted into the wells, and the HGF present was bound to the immobilized antibodies. After washing, a biotinylated antibody was added. After incubation and washing steps, a streptavidin–HRP conjugate was added. After additional incubation and washing steps a substrate solution was added. The development was stopped by the addition of HCL, and the absorbance was measured in a SpectraMax 250 (Molecular Devices, Sunnyvale, CA, USA). The HGF concentrations in the samples were determined by comparing the optical density of the sample with the standard curve.
The assays were calibrated against highly purified recombinant human HGF. The specificities of the assays were determined by the manufacturer, and they did not exhibit any cross-reactivity with a panel of other recombinant human and mouse cytokines.
Purification of seminal prostasomes
Seminal plasma was centrifuged at 10 000 g for 30 min to remove possible spermatozoa and cell debris. The supernatant was then ultracentrifuged at 100 000 g for 2 h to pellet prostasomes, and the pellet was resuspended in 30 mM Tris–HCl buffer, containing 130 mM NaCl, pH 7.6 (isotonic Tris–HCl buffer). The prostasome pellet was loaded on a Superdex column (GE Health Care, Uppsala, Sweden) and equilibrated with the isotonic Tris–HCl buffer to separate the prostasomes from an amorphous substance [34]. Fractions were collected every 20 min at a flow rate of 4 ml/hour, and the prostasome-containing fractions were identified by elevated absorbances at both 260 nm and 280 nm. The column-purified prostasomes were ultracentrifuged at 100 000 g for 2 h to pellet the prostasomes. The pellet was resuspended in Phosphate-Buffered Saline (PBS) (0.15 M NaCl, 0.01 M Na2HPO4, 0.002 M NaH2PO4, pH 7.4), and the concentration on a protein basis was adjusted to 2 mg/ml according to quantification with an ESL protein kit (Roche Diagnostics, Mannheim, Germany).
Prostasomal bound HGF
100 uL Purified prostasomes diluted 1:1000 in PBS were added to microtiter wells and incubated overnight at Room Temperature (RT). The wells were washed three times with PBS-0.05%Tween 20 and the plates were blocked with 40 mg bovine BSA/mL in PBS for 2 h at RT. The same biotinylated antibody as for the HGF assay was added. After incubation and washing steps, the streptavidin–HRP conjugate was added. After new incubation and washing steps the substrate solution was added. The development was stopped by the addition of HCL, and the absorbance was measured in a SpectraMax 250.
Statistical analyses
Mann-Whitney comparison was used to compare the seminal plasma and blood plasma values (STATISTICA; StatSoft Inc., Tulsa, OK, USA). Spearman rank correlation was used for the estimation of correlations between HGF levels and the number of sperm cells or the age of the patient.
Results
HGF levels in seminal plasma and blood plasma
All subjects had readily detectable levels of HGF in their seminal plasmas and blood plasma samples. The median age of the seminal fluid donors was 34 years. The median seminal plasma HGF was approximately five times higher than the levels found in blood plasma (5717.5 pg/mL (95% confidence interval (CI) 4736.3 to 7011.3) vs. 1124.6 pg/mL (95% CI 704.5-1523.8). The difference between seminal plasma and blood plasma was significant, p < 0.0001 (Figure 1).
Associations between seminal HGF concentrations and sperm cell counts
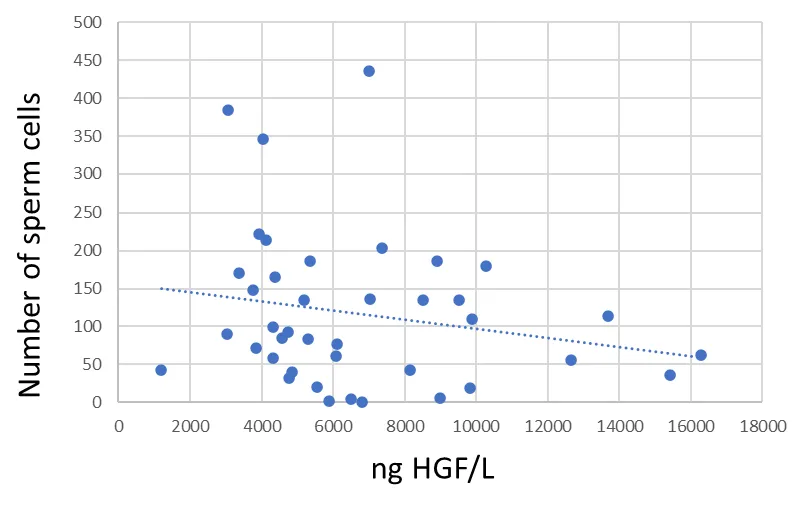
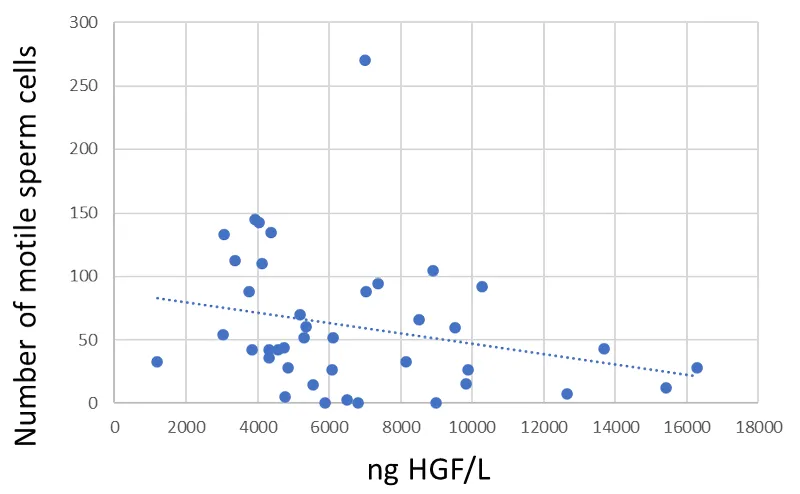
The Spearman rank correlation between seminal fluid HGF and total number of sperm cells was not significant (R =-0.228, p = 0.157) (Figure 2). There was a significant negative Spearman rank correlation between seminal fluid HGF and motile sperm cells (R =-0.352, p = 0.026) (Figure 3).
Association between seminal HGF concentrations and age of the seminal fluid donor
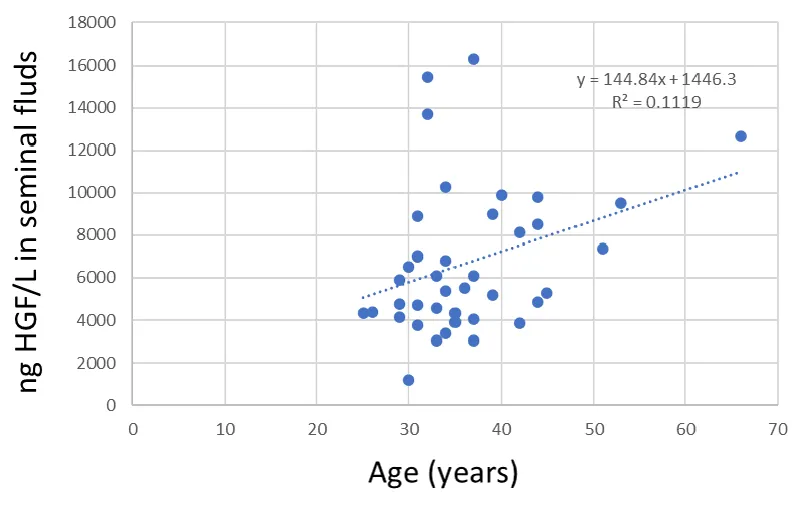
There was a significant positive correlation between the age of the seminal fluid donor and HGF values (r = 0.335, p = 0.034) (Figure 4).
Prostasomal bound HGF
In comparison with wells only blocked with BSA the prostasome-containing wells gave a significantly higher value when probed with the anti-HGF antibody.
Discussion
There are several published studies on the role of HGF in the female reproductive organs, but the information on HGF in the male reproductive organs is very limited. This is the first study quantifying HGF levels in seminal plasma, comparing it with blood plasma levels and showing that HGF is bound to prostasomes. The levels in seminal plasma are approximately five-fold higher than the levels found in blood plasma.
There was a negative correlation between seminal plasma HGF values and mobile sperm cell count. This could be explained by a higher concentration of HGF in the prostate than in the testis. A lower proportion of fluids from the testis should in theory lead to lower sperm counts but a higher seminal fluid HGF value. This is supported by one of the seminal fluid donors that had no sperm cells at all while the seminal plasma HGF value was 6811 pg/mL which was higher than the median value for seminal fluid samples. These results are not in agreement with one previous study investigating HGF in seminal plasma with regards to male fertility [35]. Wiltshire et al showed non-significant differences between group comparisons based on sperm function. They did however not perform any correlation analysis as performed in this study [35] which might explain the difference in results. Our findings are supported by tissue protein distribution data from the human protein atlas website (https://www.proteinatlas.org/ENSG00000019991-HGF/summary/rna). The highest HGF RNA levels were found in the female placenta. In the male reproductive system, the highest HGF RNA values were found in the prostate followed by the seminal vesicles, epididymis, and testis.
Seminal fluid HGF may influence the endometrial cells as an effect of unprotected sexual intercourse [29]. The presence of HGF on the surface of highly purified prostasomes indicates that HGF is bound to the prostasomes. Prostasomes adhere to sperm cells and may fuse with them, as shown by free zone electrophoresis [36] and fluorescence microscopy [37]. Previous work on HGF in seminal plasma did not investigate prostasomal-bound HGF [35]. Prostasomal-bound HGF may bind to the sperm cells and migrate together with the sperm cell to the oocyte. Seminal HGF may therefore have effects not only in the male but also in the female reproductive tract.
The HGF values were positively correlated with the age of the seminal plasma donors.
The binding of HGF to the HGF receptor causes the activation of numerous signaling pathways [38,39]. The HGF receptor is overexpressed in primary prostate cancers, and increased expression is also seen in bone metastases [40]. Thus, the HGF-HGF receptor interaction plays an important role in prostate cancer progress. Prostate cancer is the most common malignancy in men, and the tumor is mainly found in elderly males [41,42]. The positive association between age and seminal plasma HGF levels could possibly contribute to this.
Conclusion
The study shows increased levels of seminal plasma HGF in comparison with blood plasma levels. The seminal plasma HGF is not derived from the testis but rather from the prostate. The seminal plasma HGF is bound to prostasomes and may thus affect not only the male but also the female reproductive tract. Further studies are warranted to study if there are associations between seminal plasma HGF levels with fertility or prostate cancer development.
Authors’ contributions
Conceptualization, AL, LC, RK, JH and TKK; methodology, AL, RK; validation, AL, LC, TKK; formal analysis, RK, JH; investigation, AL, LC, RK, JH, TKK; resources, AL, JH, TKK; writing-original draft preparation, AL, TKK; writing-review and editing, AL, LC, RK, JH, TKK; supervision, AL, LC, TKK; project administration, AL, LC, TKK; funding acquisition, AL, TKK. All authors have read and agreed to the published version of the manuscript. AL, LC, RK, JH, TKK.
Funding
This research was funded by Uppsala University Hospital Research Fund.
Availability of data and materials
The authors declare that all supporting data are available within the article.
Declarations
Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki, and the study was approved by the ethical committee at Uppsala University (01-367). The ethical permit limited the patient information to age and sex and the use of surplus materials from clinical samples.
- Lyons HE, Arman BM, Robertson SA, Sharkey DJ. Immune regulatory cytokines in seminal plasma of healthy men: A scoping review and analysis of variance. Andrology. 2023 Mar 9. doi: 10.1111/andr.13424. Epub ahead of print. PMID: 36891953.
- Kumar N, Singh NK. "Emerging role of Novel Seminal Plasma Bio-markers in Male Infertility: A Review". Eur J Obstet Gynecol Reprod Biol. 2020 Oct;253:170-179. doi: 10.1016/j.ejogrb.2020.08.015. Epub 2020 Aug 24. PMID: 32871440.
- Schjenken JE, Robertson SA. The Female Response to Seminal Fluid. Physiol Rev. 2020 Jul 1;100(3):1077-1117. doi: 10.1152/physrev.00013.2018. Epub 2020 Jan 30. PMID: 31999507.
- World Health Organization (2021) WHO laboratory manual for the examination and processing of human semen, 6th ed. World Health Organization. https://apps.who.int/iris/handle/10665/343208
- Ata B, Abou-Setta AM, Seyhan A, Buckett W. Application of seminal plasma to female genital tract prior to embryo transfer in assisted reproductive technology cycles (IVF, ICSI and frozen embryo transfer). Cochrane Database Syst Rev. 2018 Feb 28;2(2):CD011809. doi: 10.1002/14651858.CD011809.pub2. PMID: 29489026; PMCID: PMC6491093.
- Robertson SA, Moldenhauer LM, Green ES, Care AS, Hull ML. Immune determinants of endometrial receptivity: a biological perspective. Fertil Steril. 2022 Jun;117(6):1107-1120. doi: 10.1016/j.fertnstert.2022.04.023. PMID: 35618356.
- Motrich RD, Salazar FC, Breser ML, Mackern-Oberti JP, Godoy GJ, Olivera C, Paira DA, Rivero VE. Implications of prostate inflammation on male fertility. Andrologia. 2018 Dec;50(11):e13093. doi: 10.1111/and.13093. PMID: 30569650.
- Motrich RD, Breser ML, Molina RI, Tissera A, Olmedo JJ, Rivero VE. Patients with chronic prostatitis/chronic pelvic pain syndrome show T helper type 1 (Th1) and Th17 self-reactive immune responses specific to prostate and seminal antigens and diminished semen quality. BJU Int. 2020 Sep;126(3):379-387. doi: 10.1111/bju.15117. Epub 2020 Aug 4. PMID: 32437049.
- Chen J, Chen J, Fang Y, Shen Q, Zhao K, Liu C, Zhang H. Microbiology and immune mechanisms associated with male infertility. Front Immunol. 2023 Feb 21;14:1139450. doi: 10.3389/fimmu.2023.1139450. PMID: 36895560; PMCID: PMC9989213.
- Bhargava M, Joseph A, Knesel J, Halaban R, Li Y, Pang S, Goldberg I, Setter E, Donovan MA, Zarnegar R, et al. Scatter factor and hepatocyte growth factor: activities, properties, and mechanism. Cell Growth Differ. 1992 Jan;3(1):11-20. PMID: 1534687.
- Sugie S, Mukai S, Yamasaki K, Kamibeppu T, Tsukino H, Kamoto T. Plasma macrophage-stimulating protein and hepatocyte growth factor levels are associated with prostate cancer progression. Hum Cell. 2016 Jan;29(1):22-9. doi: 10.1007/s13577-015-0123-5. Epub 2015 Aug 7. PMID: 26250899.
- Stewart F. Roles of mesenchymal-epithelial interactions and hepatocyte growth factor-scatter factor (HGF-SF) in placental development. Rev Reprod. 1996 Sep;1(3):144-8. doi: 10.1530/ror.0.0010144. PMID: 9414451.
- Cross JC, Simmons DG, Watson ED. Chorioallantoic morphogenesis and formation of the placental villous tree. Ann N Y Acad Sci. 2003 May;995:84-93. doi: 10.1111/j.1749-6632.2003.tb03212.x. PMID: 12814941.
- Verze P, Cai T, Lorenzetti S. The role of the prostate in male fertility, health and disease. Nat Rev Urol. 2016 Jul;13(7):379-86. doi: 10.1038/nrurol.2016.89. Epub 2016 Jun 1. PMID: 27245504.
- Jodar M, Soler-Ventura A, Oliva R; Molecular Biology of Reproduction and Development Research Group. Semen proteomics and male infertility. J Proteomics. 2017 Jun 6;162:125-134. doi: 10.1016/j.jprot.2016.08.018. Epub 2016 Aug 26. PMID: 27576136.
- Depuydt CE, Bosmans E, Zalata A, Schoonjans F, Comhaire FH. The relation between reactive oxygen species and cytokines in andrological patients with or without male accessory gland infection. J Androl. 1996 Nov-Dec;17(6):699-707. PMID: 9016401.
- Depuydt CE, Zalata A, de Potter CR, van Emmelo J, Comhaire FH. The receptor encoded by the human C-MET oncogene is expressed in testicular tissue and on human spermatozoa. Mol Hum Reprod. 1996 Jan;2(1):2-8. doi: 10.1093/molehr/2.1.2. PMID: 9238650.
- Depuydt CE, Zalata A, Falmagne JB, Bosmans E, Comhaire FH. Purification and characterization of hepatocyte growth factor (HGF) from human seminal plasma. Int J Androl. 1997 Oct;20(5):306-14. doi: 10.1046/j.1365-2605.1997.00074.x. PMID: 16130275.
- Depuydt CE, De Potter CR, Zalata A, Baekelandt E, Bosmans E, Comhaire FH. Levels of hepatocyte growth factor/scatter factor (HGF/SF) in seminal plasma of patients with andrological diseases. J Androl. 1998 Mar-Apr;19(2):175-82. PMID: 9570740.
- Travaglino A, Raffone A, Saccone G, Migliorini S, Maruotti GM, Esposito G, Mollo A, Martinelli P, Zullo F, D'Armiento M. Placental morphology, apoptosis, angiogenesis and epithelial mechanisms in early-onset preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2019 Mar;234:200-206. doi: 10.1016/j.ejogrb.2018.12.039. Epub 2019 Jan 14. PMID: 30721786.
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995 Feb 23;373(6516):702-5. doi: 10.1038/373702a0. PMID: 7854453.
- Ma Y, Yu X, Li YX, Wang YL. HGF/c-Met signaling regulates early differentiation of placental trophoblast cells. J Reprod Dev. 2021 Apr 21;67(2):89-97. doi: 10.1262/jrd.2020-107. Epub 2021 Jan 15. PMID: 33455972; PMCID: PMC8075731.
- Huang HS, Chen PC, Chu SC, Lee MH, Huang CY, Chu TY. Ovulation sources coagulation protease cascade and hepatocyte growth factor to support physiological growth and malignant transformation. Neoplasia. 2021 Nov;23(11):1123-1136. doi: 10.1016/j.neo.2021.09.006. Epub 2021 Oct 21. PMID: 34688971; PMCID: PMC8550993.
- Mi X, Jiao W, Yang Y, Qin Y, Chen ZJ, Zhao S. HGF Secreted by Mesenchymal Stromal Cells Promotes Primordial Follicle Activation by Increasing the Activity of the PI3K-AKT Signaling Pathway. Stem Cell Rev Rep. 2022 Jun;18(5):1834-1850. doi: 10.1007/s12015-022-10335-x. Epub 2022 Jan 28. PMID: 35089464; PMCID: PMC9209380.
- Stepanjuk A, Koel M, Pook M, Saare M, Jääger K, Peters M, Krjutškov K, Ingerpuu S, Salumets A. MUC20 expression marks the receptive phase of the human endometrium. Reprod Biomed Online. 2019 Nov;39(5):725-736. doi: 10.1016/j.rbmo.2019.05.004. Epub 2019 May 11. PMID: 31519421.
- Kreicberga I, Junga A, Pilmane M. Investigation of HoxB3 and Growth Factors Expression in Placentas of Various Gestational Ages. J Dev Biol. 2021 Dec 23;10(1):2. doi: 10.3390/jdb10010002. PMID: 35076557; PMCID: PMC8788416.
- Li G, Wang Y, Cao G, Ma Y, Li YX, Zhao Y, Shao X, Wang YL. Hypoxic stress disrupts HGF/Met signaling in human trophoblasts: implications for the pathogenesis of preeclampsia. J Biomed Sci. 2022 Feb 3;29(1):8. doi: 10.1186/s12929-022-00791-5. PMID: 35114998; PMCID: PMC8815204.
- Suryawanshi H, Max K, Bogardus KA, Sopeyin A, Chang MS, Morozov P, Castano PM, Tuschl T, Williams Z. Dynamic genome-wide gene expression and immune cell composition in the developing human placenta. J Reprod Immunol. 2022 Jun;151:103624. doi: 10.1016/j.jri.2022.103624. Epub 2022 Apr 16. PMID: 35490534.
- Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, Masuzaki H. Effect of human seminal fluid on the growth of endometrial cells of women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010 Apr;149(2):204-9. doi: 10.1016/j.ejogrb.2009.12.022. Epub 2010 Jan 25. PMID: 20092935.
- Varkaris A, Corn PG, Gaur S, Dayyani F, Logothetis CJ, Gallick GE. The role of HGF/c-Met signaling in prostate cancer progression and c-Met inhibitors in clinical trials. Expert Opin Investig Drugs. 2011 Dec;20(12):1677-84. doi: 10.1517/13543784.2011.631523. Epub 2011 Oct 28. PMID: 22035268; PMCID: PMC4020016.
- Moosavi F, Giovannetti E, Saso L, Firuzi O. HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers. Crit Rev Clin Lab Sci. 2019 Dec;56(8):533-566. doi: 10.1080/10408363.2019.1653821. Epub 2019 Sep 12. PMID: 31512514.
- Ricci G, Catizone A. Pleiotropic Activities of HGF/c-Met System in Testicular Physiology: Paracrine and Endocrine Implications. Front Endocrinol (Lausanne). 2014 Apr 3;5:38. doi: 10.3389/fendo.2014.00038. PMID: 24772104; PMCID: PMC3982073.
- Carlsson L, Ronquist G, Eliasson R, Egberg N, Larsson A. Flow cytometric technique for determination of prostasomal quantity, size and expression of CD10, CD13, CD26 and CD59 in human seminal plasma. Int J Androl. 2006 Apr;29(2):331-8. doi: 10.1111/j.1365-2605.2005.00601.x. PMID: 16533355.
- Stegmayr B, Ronquist G. Promotive effect on human sperm progressive motility by prostasomes. Urol Res. 1982;10(5):253-7. doi: 10.1007/BF00255932. PMID: 6219486.
- Wiltshire EJ, Flaherty SP, Couper RT. Hepatocyte growth factor in human semen and its association with semen parameters. Hum Reprod. 2000 Jul;15(7):1525-8. doi: 10.1093/humrep/15.7.1525. PMID: 10875860.
- Ronquist G, Nilsson BO, Hjertën S. Interaction between prostasomes and spermatozoa from human semen. Arch Androl. 1990;24(2):147-57. doi: 10.3109/01485019008986874. PMID: 2327824.
- Ronquist GK, Larsson A, Ronquist G, Isaksson A, Hreinsson J, Carlsson L, Stavreus-Evers A. Prostasomal DNA characterization and transfer into human sperm. Mol Reprod Dev. 2011 Jul;78(7):467-76. doi: 10.1002/mrd.21327. Epub 2011 Jun 2. PMID: 21638509.
- Lail-Trecker M, Gulati R, Peluso JJ. A role for hepatocyte growth factor/scatter factor in regulating normal and neoplastic cells of reproductive tissues. J Soc Gynecol Investig. 1998 May-Jun;5(3):114-21. doi: 10.1016/s1071-5576(97)00111-1. PMID: 9614639.
- Cecchi F, Lih CJ, Lee YH, Walsh W, Rabe DC, Williams PM, Bottaro DP. Expression array analysis of the hepatocyte growth factor invasive program. Clin Exp Metastasis. 2015 Oct;32(7):659-76. doi: 10.1007/s10585-015-9735-0. Epub 2015 Aug 1. PMID: 26231668; PMCID: PMC7709798.
- Whang YM, Jung SP, Kim MK, Chang IH, Park SI. Targeting the Hepatocyte Growth Factor and c-Met Signaling Axis in Bone Metastases. Int J Mol Sci. 2019 Jan 17;20(2):384. doi: 10.3390/ijms20020384. PMID: 30658428; PMCID: PMC6359064.
- Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019 Dec 1;5(12):1749-1768. doi: 10.1001/jamaoncol.2019.2996. Erratum in: JAMA Oncol. 2020 Mar 1;6(3):444. Erratum in: JAMA Oncol. 2020 May 1;6(5):789. Erratum in: JAMA Oncol. 2021 Mar 1;7(3):466. PMID: 31560378; PMCID: PMC6777271.
- Mao C, Ding Y, Xu N. A Double-Edged Sword Role of Cytokines in Prostate Cancer Immunotherapy. Front Oncol. 2021 Nov 16;11:688489. doi: 10.3389/fonc.2021.688489. PMID: 34868907; PMCID: PMC8635015.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
