Global Journal of Fertility and Research
Effects of prepubertal to peripubertal exposure of triclosan on the reproductive health of the young male laboratory mice
Shobha Raj1,2 and Poonam Singh2*
2Zoology Section, MMV, Banaras Hindu University, Varanasi - 221005, India
Cite this as
Raj S, Singh P (2023) Effects of prepubertal to peripubertal exposure of triclosan on the reproductive health of the young male laboratory mice. Glob J Fertil Res 8(1): 001-007. DOI: 10.17352/gjfr.000022Copyright
© 2023 Raj S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Over the last few decades, a massive increase in environmental toxicants has played a significant role in causing hindrance in the process of sexual maturity, leading to impaired reproductive health. Several toxicants are existing in the environment because of rapid industrialization, agricultural activities, and urbanization that act as endocrine disrupting chemicals (EDCs). Triclosan (TCS; 2,4,4’-trichloro-2’-hydroxydiphenyl ether) is one of the EDCs, acts as an antimicrobial chemical, and is used widely in personal care products and several commercial preparations as preservatives. It is found to interfere with normal reproduction and sexual maturity. The present study is therefore, aimed to investigate the impact of prepubertal to peripubertal exposure of TCS (300 and 600 mg/kg BW/day from PND 22 to PND 63) to observe the age of the onset of puberty, weights of the reproductive organs, alterations in the levels of testicular cholesterol, serum testosterone, and histopathology of the testis in the young laboratory mice. TCS exposure caused a significant delay in the age of prepuce separation, an indicator of the onset of puberty. Its exposure from PND 22 to PND 63 resulted in significant reductions in the weights of the testis, epididymis, and seminal vesicle, levels of testicular cholesterol, and serum testosterone in the young mice at PND 64. Testicular histology of such mice also showed regressive changes in the seminiferous tubules, indicating the interference of TCS in the process of spermatogenesis. The findings of the present study, therefore, reveal that TCS delays the age of onset of puberty and interferes with the endocrine functions of the testis.
Abbreviations
ANOVA: Analysis of Variance; BW: Body Weight; CNS: Central Nervous System; CPCSEA: Committee for the Purpose of Control and Supervision of Experiments on Animals; EDCs: Endocrine Disrupting Chemicals; ELISA: Enzyme-Linked Immunosorbent Assay; EPA: Environmental Protection Agency; Fig: Figure; GSI: Gonado Somatic Index; PPS: Prepuce Separation; PND: Postnatal Day; TCS: Triclosan
Introduction
The critical time period of physical maturation i.e., gaining sexual maturity and becoming capable of reproduction is generally referred to as puberty [1]. It is achieved by the maturation of multiple organs and major changes in the Central Nervous System (CNS) and psychosocial behavior [2]. Onset of puberty usually occurs between the age of 9 years to 14 years in human males during which physical and behavioral changes take place in response to the influence of several hormones [3]. Some physical changes, considered as the external sign of the onset of puberty, are the growth of testicles (first sign), hair growth around the pubic area and the scrotum, penile enlargement, and masculine-type body growth along with other growth [3]. In rodents, prepuce separation (PPS) is one of the external indicators of the onset/induction of puberty during which the prepuce gets separated from the glans penis [4-6]. In rodents, it begins at PND 35 - 40 [5]. Puberty is considered an androgen-dependent phenomenon [4,7,8]. The transition phase of puberty is maintained/regulated by the hypothalamic-pituitary-gonadal axis [6]. Further, the onset of puberty is dependent on the genetic factor [9], however, intrauterine events, family factors, stress, socio-economic conditions, exposure to toxins, and synthetic chemicals present in the environment can also affect such phase [10]. The progression of industrialization during the last few decades has added a lot of toxins in the surroundings in the form of heavy metals, pesticides, insecticides, synthetic chemicals, etc.
The toxins and synthetic chemicals mimic the endogenous hormones produced by the endocrine glands, induce deleterious effects on the endocrine system, and thus, resulting in hormonal imbalance. Such chemicals are referred to as endocrine-disrupting chemicals (EDCs). Because of having hormone-like characteristics, EDCs may act as agonists or antagonists and affect puberty via their estrogenic/antiestrogenic/androgenic or antiandrogenic activities. Exposure to various EDCs causes a progressive increase or decrease in the age of onset of puberty. Several pesticides, fungicides, herbicides, and synthetic chemicals, acting as EDCs, are reported to affect the onset of puberty [7,11]. Among such EDCs, triclosan (TCS, 2,4,4’-trichloro-2’-hydroxydiphenyl ether, a chlorophenol), an antimicrobial synthetic chemical, is widely used in personal care products, cosmetics, toys, sports items, and several commercial preparations as preservative [12-18]. It is frequently detected in the environment, such as in urban effluent [19], and surface water and soil [20-22]. The diversity of its occurrence in the environment and usage in a variety of daily products, provide a large scope of its exposure to everyone. TCS is detected in human urine, plasma, and breast milk [23-25]. Its reproductive toxicity has been reported in adults (Raj and Singh 2018) [18,24,26-30] and in few prepubertal male rodent models [31,32] in which it adversely affects the fertility. TCS affects the pubertal development in rat pups as it has been reported to delay the age of prepuce separation from the glans penis at doses of 75, 150, and 300 mg/kg BW/day, following gestational and lactational exposure [32]. TCS has also been reported to down-regulate testosterone synthesis in the rat, exposed to the dose of 200 mg/kg BW/day during PND 23 to PND 53, however, such exposure does not affect the weights of the reproductive organs [31].
Since very limited studies have been carried out regarding the onset of the puberty and status of the reproductive health of young males following TCS exposure, commencing from prepubertal to pubertal age. Hence, the present study has been designed to investigate the impact of TCS exposure from PND 22 to PND 63 to observe the age of onset of puberty, weights of the reproductive organs, and alterations in the levels of testicular cholesterol and serum testosterone, and histopathology of the testis of the young (PND 64) laboratory mouse.
Materials and methods
Animals
Eighteen, three weeks (21 days) old, prepubertal male mice were used in this study. They were procured from the Central Animal House, Institute of Medical Science, Banaras Hindu University, Varanasi, India, and maintained under standard husbandry conditions of controlled temperature (25 ± 2 0C), light (photoperiod of 14 h light and 10 h dark) and relative humidity (60% to 70%) in polypropylene cages, with rice husk as the bedding material. Animals were maintained on pelleted food and water ad libitum. An experiment was performed in accordance with the institutional practice and within the framework of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) of Govt. of India on animal welfare. Approval from the Animal Ethical Committee (1802/GO/Re/S/15/CPC5EA), Institute of Science, Banaras Hindu University, Varanasi, India was obtained to work on the mouse model.
Chemicals
TCS (Triclosan: 98% pure) and corn oil were purchased from Otto Chemie Pvt. Ltd. Chemika – Biochemika - Reagent Chemical Company, Mumbai, India.
Experimental design and dosage
After recording the initial body weights at PND 22, the mice were divided into three groups of six each (n = 6). Mice of Group I were administered with corn oil and served as vehicle-treated control, while that of Group II and III were administered with TCS at the doses of 300 and 600 mg/kg BW/day, respectively. The doses of TCS were selected based on earlier literature and converted to mice by dose converting formula [33].
Route and duration of the administration
TCS was dissolved in the corn oil and administered orally through gavage for 42 consecutive days, i.e., from PND 22 to PND 63.
Prepuce separation (PPS)
PPS was checked visually from the day of commencement of treatment in all the groups.
Animal sacrifice and collection of reproductive organs
Twenty-four hours after the last treatment, the final body weights of the mice at PND 64 were recorded and euthanized by mild ether anesthesia, followed by decapitation. Blood was collected immediately to measure the level of testosterone. The reproductive organs viz. testis, epididymis, and seminal vesicle of both sides were dissected out, blotted free of blood, and processed for the following studies:
Reproductive organs weight analyses
Wet weights of the testis, epididymis, and seminal vesicle were recorded to calculate the Gonado-Somatic Index (GSI) by using the following formula:
GSI = (Gonad weight / total body weight) ×100.
Histology of the testis
Bouin’s fixed testis was dehydrated in graded series of alcohol, cleared in xylene, and embedded in paraffin wax. Sections of 5 μm thickness were cut from each testis. The sections were dehydrated in graded series of alcohol and stained with Periodic Acid Schiff reagent followed by counterstaining with Ehrlich’s Haematoxylin.
Biochemical estimation of the level of cholesterol
The level of cholesterol was measured in the testis by using the commercial diagnostic kit, purchased from ERBA Diagnostics Mannheim GmbH Mallaustr, 69 - 73, D-68219, Mannheim/Germany.
Serum testosterone assay
The level of serum testosterone was measured by following the instructions provided in the testosterone ELISA kit {Diametra, Foligno (PG)-Italy}.
Statistical analyses
The values were represented as mean ± S.E. in each group. All the data were analyzed statistically by one-way ANOVA followed by Newman-Keul’s test for comparison of the groups. The body weight of the animals was analyzed by using Student’s t-test. Values were considered significant at p < 0.05.
Results
Prepuce Separation (PPS)
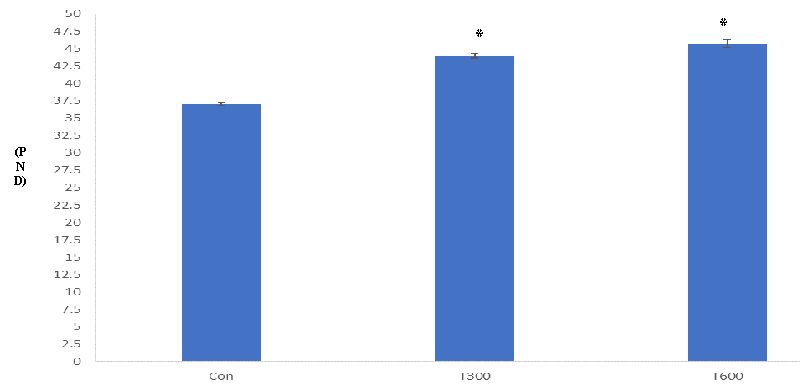
PPS in the control mice was observed at PND 33 while it appeared at PND 44 and PND 45.67 in the mice exposed to TCS at the doses of 300 and 600 mg/kg BW/day, respectively (Figure 1).
Body weight
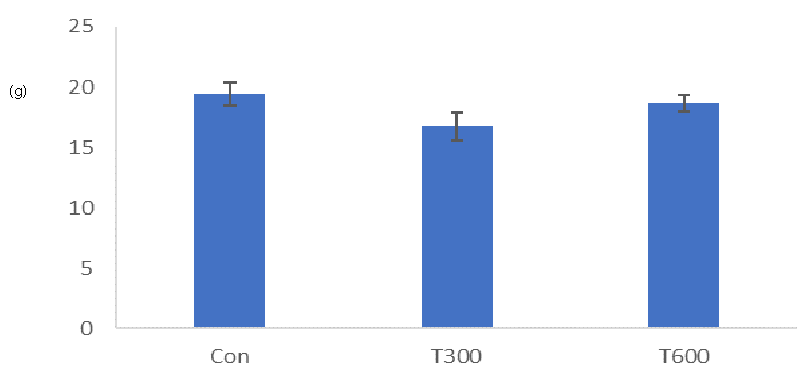
Triclosan exposure at both doses (300 and 600 mg/kg BW/day) did not affect the final body weights of the treated mice sacrificed at PND 64, as compared with the final body weights of the age-matched control (Figure 2).
Reproductive organs’ weight
Significant reductions were noticed in the weights of the testis (Figure 3), epididymis (Figure 4), and seminal vesicle (Figure 5) in the TCS (300 and 600 mg/kg BW/day)-treated mice, sacrificed at PND 64, as compared with that of the age-matched control.
Testicular histology
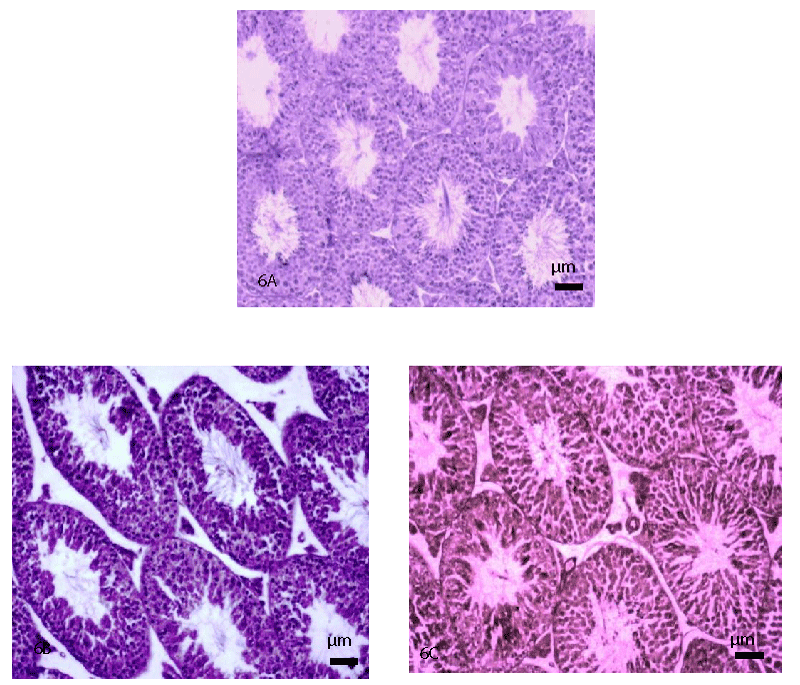
T.S. of the testis of the control mouse obtained at PND 64 showed normal histological features (Figure 6A). However, T.S. of the testis of the mice exposed with both the doses of TCS (300 and 600 mg/kg BW/day) at PND 64, showed mild histopathological alterations as indicated by the appearance of the slightly decreased height of the germinal epithelium and disorganized and detached germ cells in the seminiferous tubules (Figure 6B and 6C), as compared with that of the age-matched control (Figure 6A).
Level of testicular cholesterol
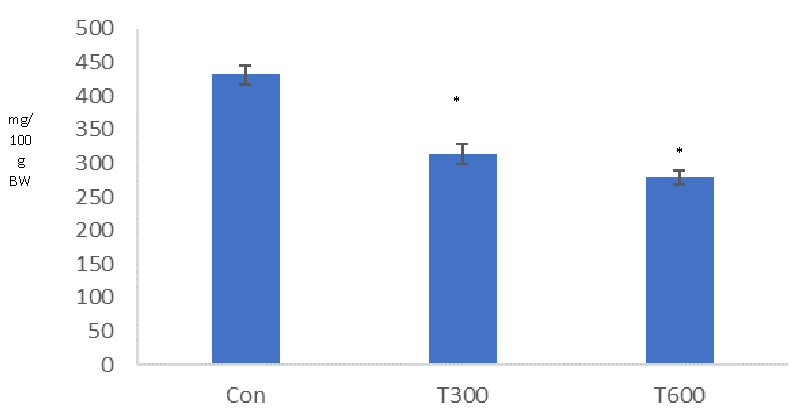
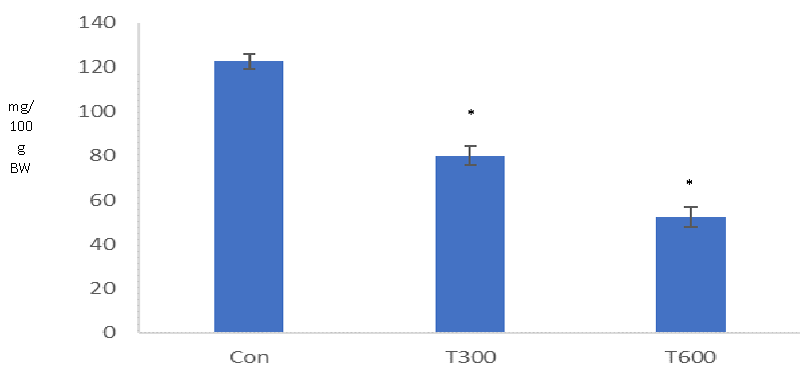
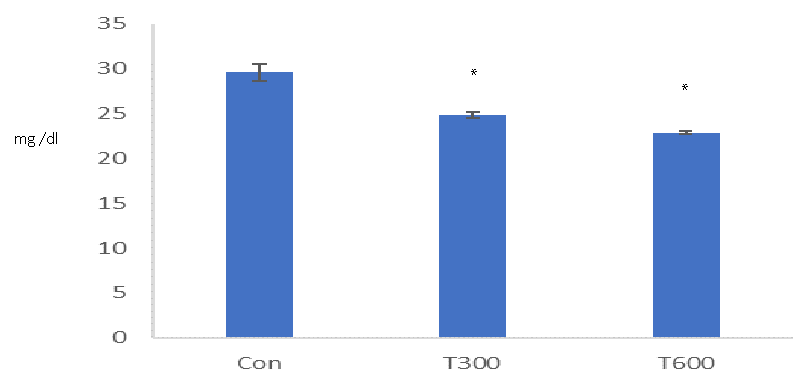
TCS, administered at both doses (300 and 600mg/kg BW/day) caused a significant decrease in the level of testicular cholesterol in the mice sacrificed at PND 64, as compared with that of the age-matched control (Figure 7).
Level of serum testosterone
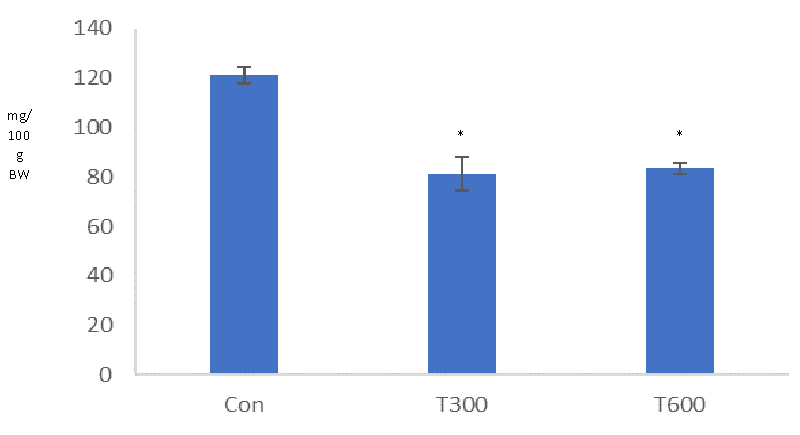
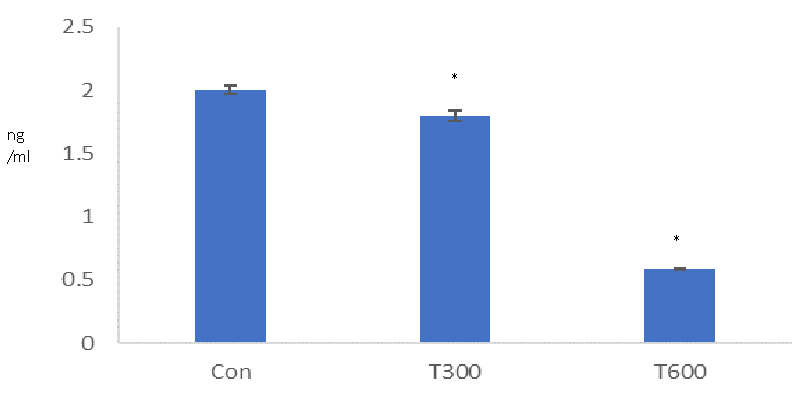
A significant reduction was found in the level of serum testosterone in the TCS-treated mice sacrificed at PND 64, as compared with that of the age-matched control (Figure 8).
Discussion
The present study has aimed to evaluate the age of onset of puberty, and alterations in the histopathology of the testis, the levels of testicular cholesterol, and serum testosterone in the young (PND 64) laboratory mice, exposed to two doses of TCS from PND 22 to PND 63. It is well documented that prepuce separation is the external sign of the onset of puberty in males [4-6].In the present study, a significant delay has been noticed in the age of PPS in both the groups of the TCS (300 and 600 mg/kg BW/day, from PND 22 to PND 63)-treated mice, as compared with that of the age-matched control. In the control mice, the age of PPS was seen at PND 33 while in both the groups of the TCS-treated mice, it was noticed at PND 44 (300 mg/kg BW/day) and PND 45.67 (600 mg/kg BW/day), respectively. Delay in PPS in such mice, thus, indicates the interference of TCS in achieving puberty on time. Machado, et al. (2017)[32] have reported similar findings in the male pups of the rat, exposed to TCS at various doses (75, 150, and 300 mg/kg BW/day) during gestational and lactational periods while in the present study delay in PPS has been noticed during prepubertal exposure. These findings, thus, indicate that TCS exposure at an early age, whether during prenatal, gestational/lactational, or prepubertal age, markedly affects the onset of puberty. Since PPS is a testosterone-dependent phenomenon (Kolho, et al. 1987), hence delay in the age of PPS may possibly be due to the unavailability of the required level of testosterone in the TCS- treated mice at PND 44 and PND 45.67, in contrast to that of the control.
Body weight is the initial indicator of good health. In the present study no significant change has been noticed in the final body weight of the TCS (300 and 600 mg/kg BW/day)-treated mice sacrificed at PND 64, as compared with that of the age-matched controls. A similar finding has been reported by Machado, et al. [32] in 60 days old male off-springs of the rat, following exposure to TCS (75, 150, and 300 mg/kg BW/day), though during gestational and lactational periods.
Reproductive organs’ weight is the primary indicator of any changes taking place in the organs after an experimental exposure. It is well documented that the weight of the testis is the primary indicator of evaluating spermatogenic activity in the organ [34]. In the present study, testicular weight was significantly decreased in the mice exposed to both doses of TCS (300 and 600 mg/kg BW/day) from PND 22 to PND 63. However, Zorrilla, et al. [31], have reported no significant alteration in the weight of the testis of the rat exposed to different doses of TCS (3, 30, 100, 200, and 300 mg/kg BW/day) from PND 23 to PND 53.
Similarly in the present study, a significant reduction has been noticed in the epididymal weight of the mice exposed to both the doses of TCS (300 and 600 mg/kg BW/day) from PND 22 to PND 63. However, findings of Zorrilla, et al. [31] have reported no alteration in the weight of the epididymis in the rat, exposed to various doses of TCS (3, 30, 100, 200, and 300 mg/kg BW/day) from PND 23 to PND 53. Likewise, Machado, et al. [32] have reported unaltered weight of the epididymis in the 60 days old offspring of the rat, exposed to various doses of TCS (75, 150, and 300 mg/kg BW/day) though, during gestational and lactational periods. The discrepancy between the findings of the present study and that of Zorrilla, et al. [31] regarding weights of the testis and epididymis may be due to the duration of the exposure of TCS and differential responses of the two genera of the rodent.
The weight of the seminal vesicle is androgen-dependent and may show changes in endocrine functions or sperm functions following any treatment [35,36]. In the present study, the weight of the seminal vesicle exhibited a significant decrease in the mice exposed to both doses of TCS from PND 22 to PND 63. A similar finding has been reported by Zorilla, et al. [31] in rats, exposed to various doses of TCS (3, 30, 100, 200, and 300 mg/kg BW/day) from PND 23 to PND 53. Machado, et al. [32] have also reported significantly decreased weight of the seminal vesicle in 60 days old offspring of the rat exposed to TCS, at the doses of 150 and 300 mg/kg BW/day, during gestational and lactational periods; though, at the dose of 75 mg/kg BW/day, these authors reported non-significantly reduced weight of the seminal vesicle. The dependency of the weights of the reproductive organs on testosterone is well documented [34-37]. Hence, the significantly reduced level of testosterone found in the TCS-exposed mice at PND 64 is the possible cause of reductions in the weights of the testis, epididymis, and seminal vesicle. Significant reductions in the weights of the testis and epididymis are also attributed to the depletion in the germ cell population (Predes, et al. 2010) as a consequence of which the sperm content also gets reduced.
In the present study, mild histopathological alterations have been noticed in the seminiferous tubules, as indicated by the loosening of the germ cells and decreased height of germinal epithelium in the testis of the mice exposed orally with TCS (300 and 600 mg/kg BW/day) from PND 22 to PND 63. On the other hand, Zorrilla, et al. [31] have reported no alterations in the histopathology of the testis of the rat exposed orally to TCS at the doses of 3, 30, 100, and 200 mg/kg BW/day from PND 23 to PND 53. However, these authors have reported the presence of multinucleated giant cells (a marker of germ cell degeneration) within the seminiferous tubules in the testis of the rat, treated only with a high dose of TCS (300 mg/kg BW/day from PND 23 to PND 53). Significant reduction in the level of serum testosterone found in the TCS-treated mice reflects the alterations in the spermatogenic activity, thus indicating the requirement of optimal level of testosterone, essential for the maintenance of spermatogenesis [38].
A significantly reduced level of testicular cholesterol, found at PND 64 indicates the interference of TCS in cholesterol biosynthesis in the mice exposed to both doses from PND 22 to PND 63. Cholesterol synthesis begins with the uptake of lipid droplets by carrier protein (cholesterol binding protein) and is transported up to the mitochondria, within the cell.TCS has been found to destabilize the functions of the cell membrane [39] and suppress phagocytosis and pinocytosis [40,41] in the cells which could be a factor to create any hindrance in the uptake of lipid droplet for the cholesterol biosynthesis.
Significant reduction in the level of serum testosterone, found in the mice following exposure to both the doses of TCS (300 and 600 mg/kg BW/day) from PND 22to PND 63 is consistent with the finding of Zorrilla, et al. [31] who also reported same finding in the rat, exposed with TCS at the dose of 200 mg/kg BW/day from PND 23 to PND 53. It is well known that cholesterol is the precursor of biosynthesis of all sex steroids [42]. Hence, in the present study, a significant reduction in the level of cholesterol could be one of the reasons for the reduced level of serum testosterone in the TCS-treated mice.
Conclusion
On the basis of the present findings, it can be concluded that the exposure of TCS from the prepubertal to peripubertal period caused detrimental effects on the reproductive health of the young male mice as indicated by delay in the age of prepuce separation, reduced weight of the reproductive organs (testis, epididymis, and seminal vesicle), regressed histopathology of the testis, decreased synthesis of cholesterol and testosterone. However, there is a need to explore the exact molecular mechanism involved in the interference of triclosan on the various reproductive parameters from the pre-to peripubertal period.
The first author is thankful to University Grant Commission, New Delhi. Financial assistance from DST PURSE 5050 to the corresponding author is also acknowledged.
- Viner RM, Allen NB, Patton GC. Puberty, Developmental Processes, and Health Interventions. In: Bundy DAP, Silva ND, Horton S, Jamison DT, Patton GC, editors. Child and Adolescent Health and Development. 3rd ed. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 20. Chapter 9. PMID: 30212144.
- Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007 Mar 31;369(9567):1130-9. doi: 10.1016/S0140-6736(07)60366-3. PMID: 17398312.
- Breehl L, Caban O. Physiology, puberty, In StatPearls. StatPearls Publishing. 2021.
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977 Sep;17(2):298-303. doi: 10.1095/biolreprod17.2.298. PMID: 889997.
- Yoshimura S, Yamaguchi H, Konno K, Ohsawa N, Noguchi S, Chisaka A. Observation of preputial separation is a useful tool for evaluating endocrine active chemicals. Journal of toxicologic pathology. 2005; 18(3): 141-157.
- Hoffmann HM. Determination of Reproductive Competence by Confirming Pubertal Onset and Performing a Fertility Assay in Mice and Rats. J Vis Exp. 2018 Oct 13;(140):58352. doi: 10.3791/58352. PMID: 30371660; PMCID: PMC6235528.
- Deboer MD, Li Y. Puberty is delayed in male mice with dextran sodium sulfate colitis out of proportion to changes in food intake, body weight, and serum levels of leptin. Pediatr Res. 2011 Jan;69(1):34-9. doi: 10.1203/PDR.0b013e3181ffee6c. PMID: 20940665; PMCID: PMC3039692.
- Auletta CS. Handbook of toxicology. CRC Press Incorporated. 2014.
- Mantos PL, Maglogiannis I. Sensitive Patient Data Hiding using a ROI Reversible Steganography Scheme for DICOM Images. J Med Syst. 2016 Jun;40(6):156. doi: 10.1007/s10916-016-0514-5. Epub 2016 May 11. PMID: 27167526.
- Hochberg Z, Belsky J. Evo-devo of human adolescence: beyond disease models of early puberty. BMC Med. 2013 Apr 29;11:113. doi: 10.1186/1741-7015-11-113. PMID: 23627891; PMCID: PMC3639027.
- Özen S, Darcan Ş. Effects of environmental endocrine disruptors on pubertal development. J Clin Res Pediatr Endocrinol. 2011;3(1):1-6. doi: 10.4274/jcrpe.v3i1.01. Epub 2011 Feb 23. PMID: 21448326; PMCID: PMC3065309.
- Daughton CG, Ternes TA. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect. 1999 Dec;107 Suppl 6(Suppl 6):907-38. doi: 10.1289/ehp.99107s6907. PMID: 10592150; PMCID: PMC1566206.
- Davies RM. The clinical efficacy of triclosan/copolymer and other common therapeutic approaches to periodontal health. Clin Microbiol Infect. 2007 Oct;13 Suppl 4:25-9. doi: 10.1111/j.1469-0691.2007.01801.x. PMID: 17716293.
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012 Sep;153(9):4097-110. doi: 10.1210/en.2012-1422. Epub 2012 Jun 25. PMID: 22733974; PMCID: PMC3423612.
- Shyam A, Fareed N. Efficacy of mouth rinses on dental plaque and gingivitis: A randomized, double-blind, placebo-controlled clinical trial. Journal of Indian Association of Public Health Dentistry. 2014; 12(3): 157.
- Dhillon GS, Kaur S, Pulicharla R, Brar SK, Cledón M, Verma M, Surampalli RY. Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health. 2015 May 22;12(5):5657-84. doi: 10.3390/ijerph120505657. PMID: 26006133; PMCID: PMC4454990.
- Ripamonti E, Allifranchini E, Todeschi S, Bocchietto E. Endocrine disruption by mixtures in topical consumer products. Cosmetics. 2018; 5(4): 61.
- Weatherly LM, Gosse JA. Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B Crit Rev. 2017;20(8):447-469. doi: 10.1080/10937404.2017.1399306. PMID: 29182464; PMCID: PMC6126357.
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ Sci Technol. 2002 Mar 15;36(6):1202-11. doi: 10.1021/es011055j. PMID: 11944670.
- Chu S, Metcalfe CD. Simultaneous determination of triclocarban and triclosan in municipal biosolids by liquid chromatography tandem mass spectrometry. J Chromatogr A. 2007 Sep 14;1164(1-2):212-8. doi: 10.1016/j.chroma.2007.07.024. Epub 2007 Jul 21. PMID: 17692856.
- Chalew TE, Halden RU. Environmental Exposure of Aquatic and Terrestrial Biota to Triclosan and Triclocarban. J Am Water Works Assoc. 2009;45(1):4-13. doi: 10.1111/j.1752-1688.2008.00284.x. PMID: 20046971; PMCID: PMC2684649.
- Reiss R, Lewis G, Griffin J. An ecological risk assessment for triclosan in the terrestrial environment. Environ Toxicol Chem. 2009 Jul;28(7):1546-56. doi: 10.1897/08-250.1. Epub 2009 Feb 19. PMID: 19228078.
- Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 2002 Mar;46(9-10):1485-9. doi: 10.1016/s0045-6535(01)00255-7. PMID: 12002480.
- Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, Takao Y, Arizono K. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol. 2004 Apr 14;67(2):167-79. doi: 10.1016/j.aquatox.2003.12.005. PMID: 15003701.
- Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, Van Aggelen G, Helbing CC. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat Toxicol. 2006 Dec 1;80(3):217-27. doi: 10.1016/j.aquatox.2006.08.010. Epub 2006 Sep 29. Erratum in: Aquat Toxicol. 2007 Jun 5;83(1):84. PMID: 17011055.
- Kumar V, Chakraborty A, Kural MR, Roy P. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod Toxicol. 2009 Apr;27(2):177-85. doi: 10.1016/j.reprotox.2008.12.002. Epub 2008 Dec 11. PMID: 19118620.
- Ibtisham F, Nawab A, Zhao Y, Li G, Xiao M, An L. Effect of antimicrobial triclosan on the reproductive system of the male rat. Pharm Anal Acta. 2016; 7(1000516): 1-5.
- Montagnini BG, Forcato S, Pernoncine KV, Monteiro MC, Pereira MRF, Costa NO, Moreira EG, Anselmo-Franci JA, Gerardin DCC. Developmental and Reproductive Outcomes in Male Rats Exposed to Triclosan: Two-Generation Study. Front Endocrinol (Lausanne). 2021 Oct 13;12:738980. doi: 10.3389/fendo.2021.738980. PMID: 34721297; PMCID: PMC8548666.
- Maksymowicz M, Machowiec PA, Ręka G, Korzeniowska A, Leszczyk P, PiecewiczSzczęsna H. Mechanism of action of triclosan as an endocrine-disrupting chemical with its impact on human health-literature review. Journal of Pre-Clinical and Clinical Research. 2021; 15(4): 169-175.
- Malashetty VB, Hegde SS, Charantimath N. Reproductive and developmental toxicity of triclosan (TCN) in Wistar rats. Austin J Environ Toxicol. 2021; 7(2):1040.
- Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, Stoker TE. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. 2009 Jan;107(1):56-64. doi: 10.1093/toxsci/kfn225. Epub 2008 Oct 21. PMID: 18940961.
- Machado CS, de Arruda Amorim JP, Welter RW, Machado MA, Amorim EMP. Maternal exposure to triclosan cause intrauterine development restriction, delay in puberty installation, and deregulation of testicular function in rat offspring. Acta Scientiarum Health Sciences. 2019; 41:37929.
- Rajasekaran UB, Nayak US. How to choose drug dosage for human experiments based on drug dose used on animal experiments: a review. IJSS Case Rep. Rev. 2014; 1:31-32.
- Creasy DM. Pathogenesis of male reproductive toxicity. Toxicol Pathol. 2001 Jan-Feb;29(1):64-76. doi: 10.1080/019262301301418865. PMID: 11215686.
- Gonzales GF. Functional structure and ultrastructure of seminal vesicles. Arch Androl. 1989;22(1):1-13. doi: 10.3109/01485018908986745. PMID: 2653253.
- Guidelines for Reproductive Toxicity Risk Assessment. EPA/630/R-96/009 October 1996; 61(212):56274-56322.
- Arrotéia KF, Garcia PV, Barbieri MF, Justino ML, Pereira LAV. The epididymis: embryology, structure, function and its role in fertilization and infertility. In Embryology-Updates and Highlights on Classic Topics, IntechOpen. 2012.
- de Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N. Spermatogenesis. Hum Reprod. 1998 Apr;13 Suppl 1:1-8. doi: 10.1093/humrep/13.suppl_1.1. PMID: 9663765.
- Villalaín J, Mateo CR, Aranda FJ, Shapiro S, Micol V. Membranotropic effects of the antibacterial agent Triclosan. Arch Biochem Biophys. 2001 Jun 1;390(1):128-36. doi: 10.1006/abbi.2001.2356. PMID: 11368524.
- Canesi L, Ciacci C, Lorusso LC, Betti M, Gallo G, Pojana G, Marcomini A. Effects of Triclosan on Mytilus galloprovincialis hemocyte function and digestive gland enzyme activities: possible modes of action on non target organisms. Comp Biochem Physiol C Toxicol Pharmacol. 2007 Apr;145(3):464-72. doi: 10.1016/j.cbpc.2007.02.002. Epub 2007 Feb 9. PMID: 17347055.
- Matozzo V, Formenti A, Donadello G, Marin MG. A multi-biomarker approach to assess effects of Triclosan in the clam Ruditapes philippinarum. Mar Environ Res. 2012 Mar;74:40-6. doi: 10.1016/j.marenvres.2011.12.002. Epub 2011 Dec 17. PMID: 22212174.
- Hadley ME, Levine JE. Endocrinology. sixth edition. 2009.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
