Global Journal of Fertility and Research
An empirical antibiotic approach to couple infertility: Is it effective
1Urology Unit, Ospedale Infermi, AUSL Emilia Romagna, Rimini, Italy
2Urology Unit, Surgery Department, Macerata Civic Hospital, SUR Marche Area Vasta 3, Macerata Civic Hospital, Macerata, Italy
3Urologic Clinic, Marche Polytechnic University, Ancona, Italy
4Statistical Analysis, University of Macerata, Italy
Author and article information
Cite this as
Scarano P, Fabiani A, Pavia MP, Gison G (2021) An empirical antibiotic approach to couple infertility: Is it effective. Glob J Fertil Res. 2021; 6(1): 006-012. Available from: 10.17352/gjfr.000019
Copyright License
© 2021 Scarano P, et a. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Objective: To evaluate the benefit of antibiotic therapy (doxycycline, azithromycin and moxifloxacin) administered to couples with a history of infertility, suspected to suffer from an unrecognized Sexual Transmitted Disease (STI), that was not detected through standard laboratory exams. To evaluate the benefit of the treatment on the basis of improvement of seminal parameters and pregnancy, both natural and assisted.
Patients and methods: The outpatient records of 350 infertile couples, were reviewed. Amongst them 136 couples met the study inclusion criteria such as history of infertility, no male or female infertility factors, negativity of laboratory cultural exams in both male and female partners, at least two seminal parameters suggestive for infections, and thus constituted the study sample. All couples were treated with 100 mg Doxycycline (1 tablet twice daily for 15 days a month for two months), 500 mg Azithromycin (1 tablet per day for 3 days every 10 days for 2 months), 400 mg Moxifloxacin (1 tablet per day for 7 days every month for 2 months). Couples were asked to refrain from having sex during the first month of therapy. Semen analyses were performed at the beginning and end of treatment. Statistical analyses comparing seminal parameters before and after treatment were carried out.
Results: The mean age of male partners was 36,11 ± 7,03 (range 18-59), while female partners’mean age was 32,7 ± 6,33 (range 18-53). The mean duration of infertility was 3,26 ± 2,69 years. A history or current symptoms of STI was present in 27,9% of females and in 19,9 % of males, while in 11.8% of the sample both partners were symptomatic. In 10,3% of couples at least one miscarriage had occurred before initial evaluation. Before treatment, semen volume was normal in 86,8% and low in 10.3% hyperviscosity was recorded in 59,6% of cases, sperm fluidity was complete and incomplete in 91,2% and 8,8%, respectively. Leukocytospermia and agglutinations were found in 21,4% and 37,5% of subjects, respectively. The baseline sperm count was 17,3 ± 14,4 million/ml and 52,34 ± 52,88 million total, in mean. Asthenospermia was present in 69,1%. The rapid and slow motility were 11,9 ± 9,5 % and 12,1 ± 6,4% in mean, respectively. After antibiotic treatment, all parameters significantly improved compared to baseline figures (all p<0.05). In 27.2% of the sample full term pregnancies were reached in 27,2%. Pregnancies were reached after treatment in a mean time of 3,7 months.
Conclusions: Couple infertility of suspected microbial etiology may be effectively treated with an antibiotic treatment regime based on a combination of doxycycline, azithromycin and moxifloxacin, which covers the most common STI pathogens. Following a holistic couple evaluation, this treatment strategy may increase the chance of natural pregnancy and decrease the need for invasive procedures.
Couple infertility may be due to female factors, male factors or both. Abnormal semen parameters are found in approximately 50% of couples seeking infertility treatment [1]. The distribution of aetiological factors for couple infertility reveals that the cause remains unknown in 50% of cases. Over the years there has been growing scientific evidence that infections can be the main cause of couple infertility [2,3]. Male infertility is evaluated through, sperm count and sperm mobility. Less than 40 million sperm count indicates infertility, with a diagnosed infection of male genital tract being the cause of both reduced sperm count in 12%-35% of case and the most common cause of asthenospermia [4]. Moreover, the standard cultural tests often do not find the infection [5,6]. The correct sperm evaluation and an accurate patient history must be performed in order to define the presence of infection. The aim of this retrospective study was to demonstrate whether the combined empiric administration of doxycycline, azithromycin and moxifloxacin in infertile couples with negative culture tests but at least two seminal parameters indicating inflammation [7], improve sperm metrics and result in increased pregnancy rates, with or without Assisted Reproductive Techniques (ART).
Patients and methods
This was a non randomized non controlled retrospective study in which treatment was not adherent to current guidelines. The study sample consisted of 136 infertile couples that were selected from 350 consecutive assessments at our out-patient clinics. The 136 couples met on the basis of the study 5 inclusion criteria: 1) Previous infertility evaluation with a diagnosis of primary infertility; 2) Absence of any female factor of infertility; 3) Absence of any male factor of infertility; 4) Negative tests for urogenital infections in at least two consecutive semen cultures for men, and urethral swab and cervical smear for women; and 5) Presence of at least two seminal parameters indicating inflammation (oligospermia, asthenospermia, reduction of ejaculate volume, hyperviscosity, leukocytospermia, agglutinations, high pH, urogenital symptoms in male or female).
All men underwent andrological evaluation, including hormonal and genetic tests, scrotal ultrasound, in order to exclude defined causes of infertility. All women were re-evaluated to exclude a female infertility factor. The following seminal parameters were measured: semen volume, pH, sperm viscosity, sperm fluidity, sperm concentration (number of spermatozoa/ml), total sperm count, progressive sperm motility, leukocytospermia, presence of erythrocytes and agglutinations. The seminal variables were considered normal or altered on the basis of the WHO 2010 reference values [8]. Uro- genital symptoms from both partners were also recorded (e.g. dysuria, vaginal discharge, painful intercourse and/or abdominal pain, painful ejaculation, irritative lower urinary tract symptoms). Both partners in each eligible couple were treated with 100 mg doxycicline (1 tablet twice daily for 15 days a month for two months), 500 mg azythromycin (1 tablet per day for 3 days every 10 days for 2 months), 400 mg moxifloxacin (1 tablet per day for 7 days every month for 2 months). Couples were asked not to have sex during the first month of therapy and then resumed unprotected, fertilizing intercourses. Semen analyses were performed again at the end of treatment. In order to evaluate the effectiveness of the antibiotic treatment intervention, we compared baseline semen analyses measures with end of treatment semen analyses measures, using two-tailed pair-samples t-tests. The number of pregnancies in these previously infertile couples following treatment was also recorded. Informed consent for off label therapy was acquired by each patient. The statistical analyses were carried out using SPSS version 25.
Results
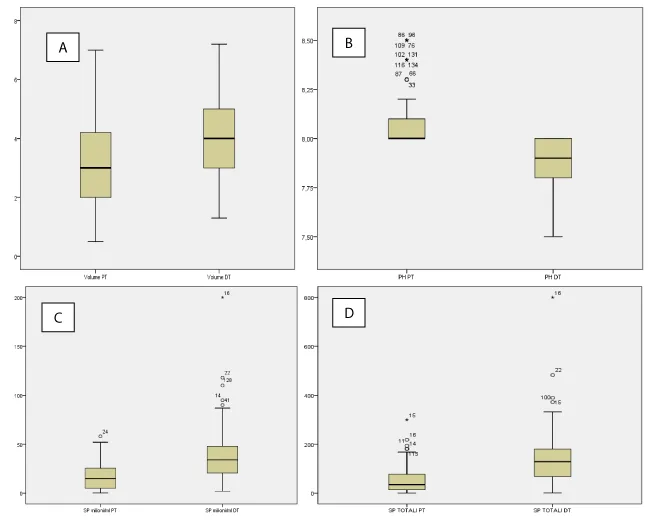
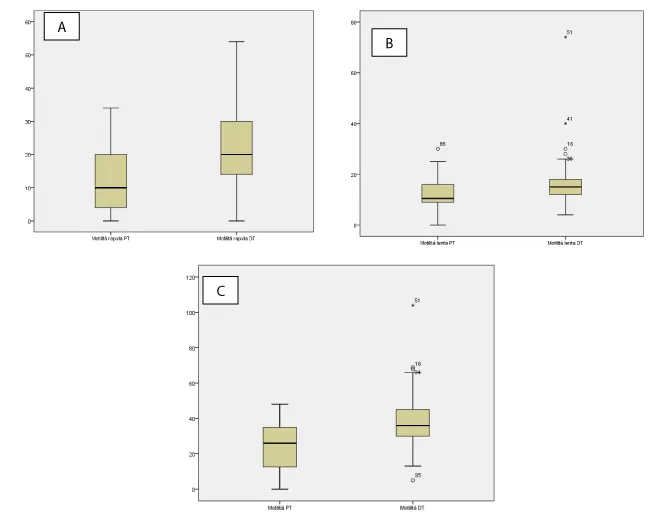
The mean age of the male partners was 36,11 ± 7,03 (range 18-59), and of female partners was 32,7 ± 6,33 (range 18-53). The difference in age between the two sexes reaches a maximum of 11 years in the case of women (older age than men’s) while 22 years in the opposite case. The mean duration of infertility was 3,26 ± 2,69 years. A history or actual symptoms of genital tract infection was present in 27,9% of female partners and in 19,9 % of male partners. In 11,8% of cases, both partners complained of uro-genital symptoms. In 10,3% of the sample, we found a history of at least one miscarriage, while 4 abortions occurred in 2 cases (1,5%). Table 1 shows the semen parameters before and after antibiotic treatment . Before the therapy, semen volume was normal in 86,8% of cases. Low semen volume was registered in 14 couples (10.3%). Hyperviscosity was found in 59,6% of evaluated male. Sperm fluidity was considered as complete in 91,2% of the sample. A leukocytes count of 0.2-1.0 x 106 WBC/ml was considered abnormal and defined as leukocytospermia. In our population, leukocytospermia was found in 21,4% of cases and agglutinations were present in 37,5% of subjects. Considering normal a pH semen ranging between 7,2 and 8,2 we found 13 cases (9.6%) of increased pH beyond the upper reference value. The baseline sperm count mean value of was 17,3 ± 14,4 million/ml and 52,34 ± 52,88 million total. It was found that 47,8% of male presented with an abnormal number of spermatozoa/ml and 47,1% with an altered total sperm count. Asthenospermia (defined as a sperm motility less than 32%) was present in 69,1% of patients. The mean value of rapid and slow motility was 11,9 ± 9,5 % and 12,1 ± 6,4%, respectively. After therapy, sperm viscosity, pH, fluidity, leukocytospermia, agglutinations significantly improved (Table 2). The semen volume reached a mean value of 4 ± 1,3 ml, while remaining altered in only 2,2% of cases. As shown by the box-plots (Figures 1,2), both mean volume and pH, sperm concentration, total sperm count and rapid or slow motility, improved significantly from baseline to after treatment. In particular, the rapid motility improvement was better than the slow, with a mean of 21,9 ± 10,8 % (pre treatment 11,9%) and 15,3 ± 7% (pre-treatment 12,1%), respectively. Overall, the global motility improved after therapy, with a score of 37,3 ± 12,9% compared to a pre-treatment mean value of 23,9 ± 13,1%). Given the differences in values between the before and the after therapy, it was decided to carry out a T-test for dependent samples. Only for the pH and the slow motility, we highlighted a low correlation index, with a value of 0,152 and 0,068, respectively, with significance levels equal to 0.77 for pH and 0.432 for slow motility. All the other pairs of parameters (Table 1) showed a correlation index higher than 0.7 except for the total sperm count which had a value equal to 0.587 but a significance level <0.05>>. The T-test, carried out on pairs of variables, showed that the differences in the means are all significant (two-tailed test with a level of significance equal to 0.05) also for those that showed low correlation indices. The confirmation of the differences due to the treatment to which the couples have undergone can be verified through the analysis of the clinical history variables. As regards the symptoms present in males, there is an improvement compared to the values recorded before the therapy (27 against 9) as well as the overall symptoms in the females that were present in 38 cases before the therapy and are zeroed after treatment. A similar result is evident with regard to couples who have joint symptoms in the two sexes (they were 16 before therapy) (Table 3). No adverse events were recorded. After antibiotic empirical treatment, 27,9% of women fell pregnant, 37 were full term pregnancies (29 naturally conceived and 8 through ART) while one was extra-uterine. The mean time from end of antibiotic therapy and pregnancy was 3,7 months (range 1-9).
Discussion
We know that 15% of couples have difficulties with conceiving within a year from trying and that in 50% of these cases a male infertility associated factor may be present and in another 30% detectable abnormalities can be found in both partners [9]. Whenever a treatable condition that may affect male fertility is diagnosed, such as hypogonadism, varicocele, immunological infertility, obstructions and infections [10], every effort should be made to correct the underlying condition with the specific recommended medical and surgical treatments [11]. However, many aspects of male factor infertility are still poorly understood, and patients often diagnosed with idiopathic infertility, are treated without an etiological basis being clearly established. The introduction of the Assisted Reproduction Technique (ART) has transformed the role of the andrologist in the couple infertility management. The relatively good fertilization and pregnancy rates achieved through ART have led to a progressive weakening in the efforts not only to establish an etiological diagnosis and improve sperm quality in vivo, but also in clinical attention to the evaluation of possible infertility factors in the male partners. In fertility centers, the andrologist is often not very involved in the management of couple infertility because it is considered that a fertile female partner may compensate for the possible male fertility problems. On the other hand, the andrologist tends to underestimate the need for holistic infertile couple evaluation. However, we believe that evaluating the fertility potential of the male partner represents an important part in the assessment of infertile couples. As andrologists we are trained to evaluate accurately the male fertility factor, and we commonly research this factor stratifying male infertility by mechanism. When we fail to clearly define a cause of infertility, we tend to diagnose idiopathic infertility, due to the lack of contextual evaluation of both partners of the infertile couple. Ideally, the male factor evaluation should be performed simultaneously with the evaluation of the female partner, but this is not done in at least 18% of cases [12], thus compromising the fertility prognosis of the couple and also missing an opportunity to improve health outcomes in male patients [13]. In addition, we found that about 20% of male patients complain of uro-genital symptoms. The contextual female partner evaluation permitted to find a 27,9% of women suffering from genital symptoms. All women after our therapy became asymptomatic. In 11,8% of cases, both partners were affected by symptoms probably related to the infective cause of infertility. Had we not taken a holistic approach to the evaluation of the infertile couple, dedicating ourselves only to the female, we would have lost the possibility of treating the sperm impairment. Moreover, in our selected population study, we found that a relatively high percentage of couples (19,1%) had already undergone ART, probably because they were considered affected by idiopathic infertility. Regardless of the negativity of the culture tests, in each couple we suspected the presence of a sexually transmitted infection (STI) responsible for sperm abnormality and, consequently, for infertility. Being unable to demonstrate the bacterial presence, we based our assumption on laboratory data and anamnesis to prescribe an empirical antibiotic therapy covering the most common pathogens of STI [4]. This study highlights the need to investigate the important role of some intracellular pathogens, such as Mycoplasmas (Mycoplasma Hominis, Ureaplasma Urealyticum and Ureaplasma Parvum) and to Chlamydia Trachomatis (Ct), in being implicated in causing infertility in a sub-group of couples. For both Mycoplasmas and Ct there are no international evidence-based management guidelines since effective treatment regimens are lacking [13]. Data from the literature suggest an association of Mycoplasma hominis and Ureaplasma Urealyticum with infertility in men [14-16] with a molecular detection that is likely to reflect the transmission to their female sexual partner [17]. Ureaplasma parvum is difficult to differentiate from Ureaplasma urealyticum, and thus, no association with male infertility has been demonstrated yet [18]. The recently updated guidelines state that Ureaplasma urealyticum is pathogenic only in high concentrations (>103 cfu/ml ejaculate). No more than 10% of samples analysed for Ureaplasma exceeds this concentration [1]. Ct is the most common sexually transmitted bacterium worldwide and, in particular, the most frequently reported STI in Europe with a number steadily increasing and asymptomatic in about 70% of cases [19,20]. The risk to infect sexual partners is high, causing long-term complications both in women and in men. Ct is difficult to detect by a conventional analysis and the DNA recombination techniques are universally accepted as the “gold standard” to evaluate his presence in biologic samples [21,22]. However, despite the extended diagnostic effort providing highly specific and sensitive methods for the detection of most of bacterial pathogens, it remains difficult to detect these pathogens or differentiate between infections, contamination and colonization of the genital tract [5]. Therefore, the negativity of the cultural tests in our study population may be accounted for by the low sensitivity and hence unreliability of current means for bacterial detection. On the basis of the results from our sample, which showed alterations of the seminal parameters, we inferred the presence of intracellular pathogens responsible for the couples’ infertility. Therefore, we treated these couples with a regime of antibiotic treatment based on our knowledge of the biological features of these bacteria. Because Mycoplasmas lack the rigid cell wall of other bacteria, they are intrinsically resistant to penicillins and cephalosporins, and other antimicrobials targeting cell wall. Mycoplasma hominis is additionally naturally resistant to clarithromycin and erythromycin but not to other type of macrolides such as josamycin. The in vitro susceptibility to doxycycline is high only for certain subtypes. Ureaplasma Urealyticum is moderately sensitive to azitromycin. In general, urogenital mycoplasmas can be difficult to eradicate in many individuals because of true antimicrobial resistance but also because of lower activity of the antimicrobials at low pH and lack of bactericidal activity [13]. In management and treatment of patient affected by Ct infection, we must take into account that Ct are only metabolically active in the host cell and therefore only targeted intracellularly by antibiotics. Thus, the antimicrobial groups effective against Ct include the macrolides, tetracyclines, quinolones, suggesting that doxycycline and azytromycin are considered to be equally effective in the treatment of Ct infections [23]. Even if doxycycline and azithromycin are the most widely prescribed drugs in Ct infections treatment and recommended as the primary approach, other fluoroquinolones such as ofloxacin or levofloxacin are suggested as alternative drugs. Moreover, although little is known about Ct survival in the presence of fluoroquinolones, it is well know that after multiple cultivation passages, resistant mutants for some fluoroquinolones were determined [23). Bébéar and co-workers confirm in their study the high in vitro activity of moxifloxacin against a significant number of Ct and urogenital mycoplasmas isolates, configuring them as a first choice for the treatment of these urogenital infections [24]. Thus, we pioneered the use of an empirical antibiotic approach with 100 mg Doxycicline (1 tablet twice daily for 15 days a month for two months), 500 mg Azythromycin (1 tablet per day for 3 days every 10 days for 2 months), 400 mg Moxifloxacin (1 tablet per day for 7 days every month for 2 months), mindful that recommended antibiotic treatment regimens do not lead to the complete infections eradication [1]. Although the available literature shows that antibiotic therapy leads to an improvement in seminal parameters, these studies are based on samples with positive cultural exams, even asymptomatic, and the antibiotic regime employed was different. Pajovic, et al, in case of pyospermia, defined as a number of leucocytes <106, treated Ct infection, in accordance with the recommendations of the European Association of Urology, with one oral dose of 1000 mg of azytromycin and Ureaplasma Urealyticum with metronidazole in a single oral dose of 2000 mg followed by erytromicin at 500 mg taken orally four times daily over a period of 7 days. Ten days after the completion of the antibiotic therapy, in 60 infertile patients they obtained an improvement in all parameters for fertile characteristics. While no statistical significance was achieved in improving sperm concentration and viability 10 days after completing the therapy, sperm concentration was significantly increased 30 days afterwards [25]. Our patients performed sperm evaluation after 30 days from the end of treatment because the increase of spermatozoa concentration or sperm viability need more time to show compared to the time lag required by the other semen parameters to improve. Ahmadi and colleagues showed that in 165 infertile males, with abnormal semen parameters, the antibiotic therapy, prescribed in case of infection, improved the semen quality [26]. Similar results were reached by the same group concerning asymptomatic Mycoplasma hominis infection treated with doxycicline, 100 mg orally twice daily for seven days [16]. Sena and colleagues demonstrated that clinical failures among men treated for Non Gonococcical Urethritis (NGU) with recommended therapies are common, especially in cases of Mycoplasma genitalium infections. In a population of 293 men treated for NGU, they revealed, at 4 weeks after therapy, a persistent Ct and Mycoplasma genitalium infections in 44% and 31% of cases respectively. Persistent Ct was detected in 23% of participants after azytromycin treatment vs 5% after doxycycline treatment; persistent Mycoplasmas was detected in 68% of participants after doxycycline vs 33% after azithromycin. They called for randomized trials exploring potentially higher doses and/or longer treatment duration of azythromycin or other drug regimens (ie moxifloxacin) as optimal therapy for management of these persistent NGU [27]. Malallah and co-workers demonstrated an improvement of sperm count and the sperm motility in infertile men with high pus cell count after minocycline 200 mg/daily for one or two weeks (28). Similarly, Carranza-Lira and colleagues treated thirteen patients with asthenozoospermia and semen analysis suggestive of infection. They concluded that antibiotic administration modified some of the spermatic parameters, particularly motility and morphology [29]. More recently, Hamada et al evaluated a population of 223 male infertile patient, identifying 61 patients with a leukocytes count of 0.2-1.0 x 106 WBC/ml. Of the 61 men, 27 were not routinely treated for this level of leukocytospermia. 34 patients were treated with an empirical antibiotic therapy (doxycycline). They obtained a 56% of resolved leukocytospermia in the therapy group, well above the spontaneous resolution rate of 25% observed in the control group. The natural pregnancy rate among the treatment group (47%) was significantly greater than that among the controls (20%) [30]. Vicari and colleagues revealed a beneficial effect of levofloxacin treatment on semen hyperviscosity and registered an improvement of sperm progressive motility [31]. In this study we found that antibiotic treatment improved seminal parameters, in particular those indicative of an infection of the seminal tract, such as viscosity, sperm agglutinations, sperm count, sperm motility and fluidification The goal of the andrologist in the management of the infertile male is to increase in the odds of natural and non-natural conception in couples. The guidelines reported that although antibiotic treatment for seminal infections might provide improvement in sperm quality, it does not necessarily enhance the probability of conception [1]. Our results have shown, that treating the infertile couple, not just a single partner, with our empiric antibiotic regimen, a 27,9% increase in pregnancy rates was achieved in our sample in a relatively short time (3,7 months on average). What is not yet clear is how long the antibiotic therapy should be. The most frequent objection to our therapeutic approach concerns the duration of the treatment because of its possible unwanted effects. However, we recorded no major adverse events. We believe that there is a need to resort to a longer antibiotic treatment regime in an attempt not so much to eradicate the STI, as to create the ideal conditions to promote a natural pregnancy or to aid ART methods. It is evident that our study has a number of methodological limitations such as its retrospective nature and the lack of a control group, which are a threat to the internal validity of the study. Moreover, the laboratory tests were not centralized. Despite these limitations, our promising study results may lead to the establishment of prospective controlled studies that will allow to standardize therapy. To approach the diagnosis it is important to also evaluate some of the seminal parameters and always consider the couple as a single entity, starting from anamnesis. We know from the scientific literature that antibiotic treatment can eliminate pathogens, but does not affect inflammatory changes and does not recover any functional and anatomical damage that may be determined. Moreover, the antibiotic treatment can improve semen quality but may not necessarily increase the likelihood of conception. However, our empirical antibiotic treatment, in selected cases with high suspicious of STI, may improve both seminal parameters and pregnancy rates. Further randomized studies are needed to confirm the effectiveness of this longer term antibiotic regime.
Conclusion
In our study we examined the effect of treating with an empiric antibiotic scheme both partners in a sample of infertile couples. In case of suspected infective etiology of couple infertility, basing on laboratory data and anamnesis, we found that the prescription of empirically antibiotic therapy with doxycycline, azythromycin and moxifloxacin, covering the most common pathogens affecting genital system, brought about improvements in seminal parameters and improved the chances of conception within months from the end of treatment. These promising results suggest the need to set up prospective controlled studies in order to standardize type and duration of treatment. We need to increase the chance of natural pregnancy and decrease the need for invasive procedures or facilitate the ART success rate, starting from a holistic couple evaluation.
- Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, et al. (2012) European Association of Urology Working Group on Male Infertility. European Association of Urology guidelines on Male Infertility: the 2012 update. Eur Urol 62: 324-332. Link: https://bit.ly/3eyDfkn
- Comhaire FH, Mahmoud AM, Depuydt CE, Zalata AA, Christophe AB (1999) Mechanisms and effects of male genital tract infection on sperm quality and fertilizing potential: the andrologist's viewpoint. Hum Reprod Update 5: 393-398. Link: https://bit.ly/3bi3V6V
- Ruggeri M, Cannas S, Cubeddu M, Molicotti P, Piras GL, et al. (2016) Bacterial agents as a cause of infertility in humans. New Microbiol 39: 206-209. Link:
- Stojanov M, Baud D, Greub G, Vulliemoz N (2018) Male infertility: the intracellular bacterial hypothesis. New Microbes New Infect 26: 37-41. Link: https://bit.ly/3uBs9Rc
- Solomon M, Henkel R (2017) Semen culture and the assessment of genitourinary tract infections. Indian J Urol 33: 188-193. Link: https://bit.ly/33yxQU5
- Levy R, Layani-Milon MP, Giscard D'Estaing S, Najioullah F, Lornage J, et al. (1999) Screening for Chlamydia trachomatis and Ureaplasma urealyticum infection in semen from asymptomatic male partners of infertile couples prior to in vitro fertilization. Int J Androl 22: 113-118. Link: https://bit.ly/3vRZ3gA
- La Vignera S, Condorelli RA, Vicari E, Salmeri M, Morgia G, et al. (2014) Microbiological investigation in male infertility: a practical overview. J Med Microbiol 63: 1-14. Link: https://bit.ly/3uMy99y
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, et al. (2010) World Health Organization reference values for human semen characteristics. Hum Reprod Update 16: 231-245. Link: https://bit.ly/3oeRHkR
- Foresta C, Bettella A, Garolla A, Ambrosini G, Ferlin A (2005) Treatment of male idiopathic infertility with recombinant human follicle-stimulating hormone: a prospective, controlled, randomized clinical study. Fertil Steril 84: 654-661. Link: https://bit.ly/3y1gsoL
- Isidori A, Latini M, Romanelli F (2005) Treatment of male infertility. Contraception 72: 314-318. Link: https://bit.ly/3heJ1t3
- Ben-Rafael Z, Farhi J, Feldberg D, Bartoov B, Kovo M, et al. (2000) Follicle-stimulating hormone treatment for men with idiopathic oligoteratoasthenozoospermia before in vitro fertilization: the impact on sperm microstructure and fertilization potential. Fertil Steril 73: 24-30. Link: https://bit.ly/3fbq87T
- Eisenberg ML, Lathi RB, Baker VL, Westphal LM, Milki AA, et al. (2013) Frequency of the male infertility evaluation: data from the national survey of family growth. J Urol 189: 1030-1034. Link: https://bit.ly/33snsxb
- Horner P, Donders G, Cusini M, Gomberg M, Jensen JS, et al. (2018) Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women? - a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol 32: 1845-1851. Link: https://bit.ly/3uuzv8Z
- Huang C, Zhu HL, Xu KR, Wang SY, Fan LQ, et al. (2015) Mycoplasma and ureaplasma infection and male infertility: a systematic review and meta-analysis. Andrology 3: 809-816. Link: https://bit.ly/3oeRYnT
- Huang C, Long X, Jing S, Fan L, Xu K, et al. (2016) Ureaplasma urealyticum and Mycoplasma hominis infections and semen quality in 19,098 infertile men in China. World J Urol 34: 1039-1044. Link: https://bit.ly/3uDJXuM
- Ahmadi MH, Mirsalehian A, Sadighi Gilani MA, Bahador A, Talebi M (2017) Asymptomatic Infection With Mycoplasma hominis Negatively Affects Semen Parameters and Leads to Male Infertility as Confirmed by Improved Semen Parameters After Antibiotic Treatment. Urology 100: 97-102. Link: https://bit.ly/3heNv31
- Mändar R, Punab M, Borovkova N, Lapp E, Kiiker R, et al. (2015) Complementary seminovaginal microbiome in couples. Res Microbiol 166: 440-447. Link: https://bit.ly/33uRJv9
- Liu J, Wang Q, Ji X, Guo S, Dai Y, et al. (2014) Prevalence of Ureaplasma urealyticum, Mycoplasma hominis, Chlamydia trachomatis infections, and semen quality in infertile and fertile men in China. Urology 83: 795-799. Link: https://bit.ly/3hgOyiO
- Mazzoli S, Cai T, Addonisio P, Bechi A, Mondaini N, et al. (2010) Chlamydia trachomatis infection is related to poor semen quality in young prostatitis patients. Eur Urol 57: 708-714. Link: https://bit.ly/3xZP9vf
- Mazzoli S, Cai T, Rupealta V, Gavazzi A, Castricchi Pagliai R, et al. (2007) Interleukin 8 and anti-chlamydia trachomatis mucosal IgA as urogenital immunologic markers in patients with C. trachomatis prostatic infection. Eur Urol 51: 1385-1393. Link: https://bit.ly/3bfOImL
- Cai T, Mazzoli S, Mondaini N, Malossini G, Bartoletti R (2010) Chlamydia trachomatis infection: the urologist’s point of view. Journal of Andrological Sciences 17: 164-168. Link:
- Keck C, Gerber-Schäfer C, Clad A, Wilhelm C, Breckwoldt M (1998) Seminal tract infections: impact on male fertility and treatment options. Hum Reprod Update 4: 891-903. Link: https://bit.ly/3hgkfbR
- Bonkat G, Bartoletti R, Bruyère F, Cai T,. Geerlings SE, et al . (2020) EAU Guidelines: urological infections. Link: https://bit.ly/3bc6s2b
- Bébéar CM, de Barbeyrac B, Pereyre S, Renaudin H, Clerc M, et al. (2008) Activity of moxifloxacin against the urogenital mycoplasmas Ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect 14: 801-805. Link: https://bit.ly/3uzZ0pf
- Pajovic B, Radojevic N, Vukovic M, Stjepcevic A (2013) Semen analysis before and after antibiotic treatment of asymptomatic Chlamydia- and Ureaplasma-related pyospermia. Andrologia 45: 266-271. Link: https://bit.ly/3ttxRTK
- Ahmadi MH, Mirsalehian A, Gilani MAS, Bahador A, Talebi M (2018) Improvement of semen parameters after antibiotic therapy in asymptomatic infertile men infected with Mycoplasma genitalium. Infection 46: 31-38. Link: https://bit.ly/3tyRjhS
- Seña AC, Lensing S, Rompalo A, Taylor SN, Martin DH, et al. (2012) Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis infections in men with nongonococcal urethritis: predictors and persistence after therapy. J Infect Dis 206: 357-365. Link: https://bit.ly/3uMTmk5
- Malallah YA, Zissis NP (1992) Effect of minocycline on the sperm count and activity in infertile men with high pus cell count in their seminal fluid. J Chemother 4: 286-289. Link: https://bit.ly/2RDSe3x
- Carranza-Lira S, Tserotas K, Morán C, Merino G, Barahona E, et al. (1998) Effect of antibiotic therapy in asthenozoospermic men associated with increased agglutination and minimal leukospermia. Arch Androl 40: 159-162. Link: https://bit.ly/33uSgx9
- Hamada A, Agarwal A, Sharma R, French DB, Ragheb A, et al. (2011) Empirical treatment of low-level leukocytospermia with doxycycline in male infertility patients. Urology 78: 1320-1325. Link: https://bit.ly/3y1hjWv
- Vicari LO, Castiglione R, Salemi M, Vicari BO, Mazzarino MC, et al. (2016) Effect of levofloxacin treatment on semen hyperviscosity in chronic bacterial prostatitis patients. Andrologia 48: 380-388. Link: https://bit.ly/3tzY4jE
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
