Global Journal of Fertility and Research
Impact of Oxidative stress on Infertility, with emphasis on infertility management strategies
Pamela Banerjee1* and Jayashree Bhattacharya2
2MD, MRCOG, FRCOG, London, UK, Director, A.H IVF & Infertility Research Centre, 853, Kalikapur Road, Kolkata 78, India
Cite this as
Banerjee P, Bhattacharya J (2019) Impact of Oxidative stress on Infertility, with emphasis on infertility management strategies. Glob J Fertil Res 4(1): 010-018. DOI: 10.17352/gjfr.000012Oxidative stress (OS) is a state of imbalance between pro-oxidants (Reactive Oxygen species and Reactive Nitrogen species) and antioxidant defences. It plays a pivotal role in infertility issues (paternal as well as maternal). As such different reproductive diseases such as Polycystic Ovary Syndrome (PCOS), Endometriosis, unexplained infertility, Impairment of Spermatozoa, Sperm Dysfunction and Sperm DNA Fragmentation (SDF), are caused, which destroys the process of reproduction. Several pregnancy complications such as miscarriage, recurrent respiratory loss (RPL), preeclampsia and intrauterine growth restriction (IUGR), are also associated to OS. It is well established that factors such as obesity, malnutrition, life style factors – smoking, alcohol and recreational drugs, environmental and occupational exposures – pesticides and endocrine disrupting chemicals, EDCs, hampers the reproductive process by setting up OS. Thus, as an infertility management strategy, procedures such as antioxidant supplementation and Assisted Reproductive Techniques (ART), are to be undertaken. There is also a wide scope for research in the field of EDCs linked to oxidative stress, which can be a hope to further elucidate the path of reproduction.
Abbreviations
OS: Oxidative Stress; ROS: Reactive Oxygen Species; PCOS: Polycystic Ovary Syndrome; SDF: Sperm DNA Fragmentation; RPL: Recurrennt Respiratory Loss; IUGR: Intrauterine Growth Restriction; EDCs: Endocrine Disrupting Chemicals; ART: Assisted Reproductive Techniques; IVF: Invitro Fertilization; ICSI: Intracytoplasmic Sperm Injection; IUI: Intrauterine Insemination; MDA: Malonaldialdehyde; PBBs: Polychlorinated Biphenyls; BPA: Bisphenol A.
Introduction
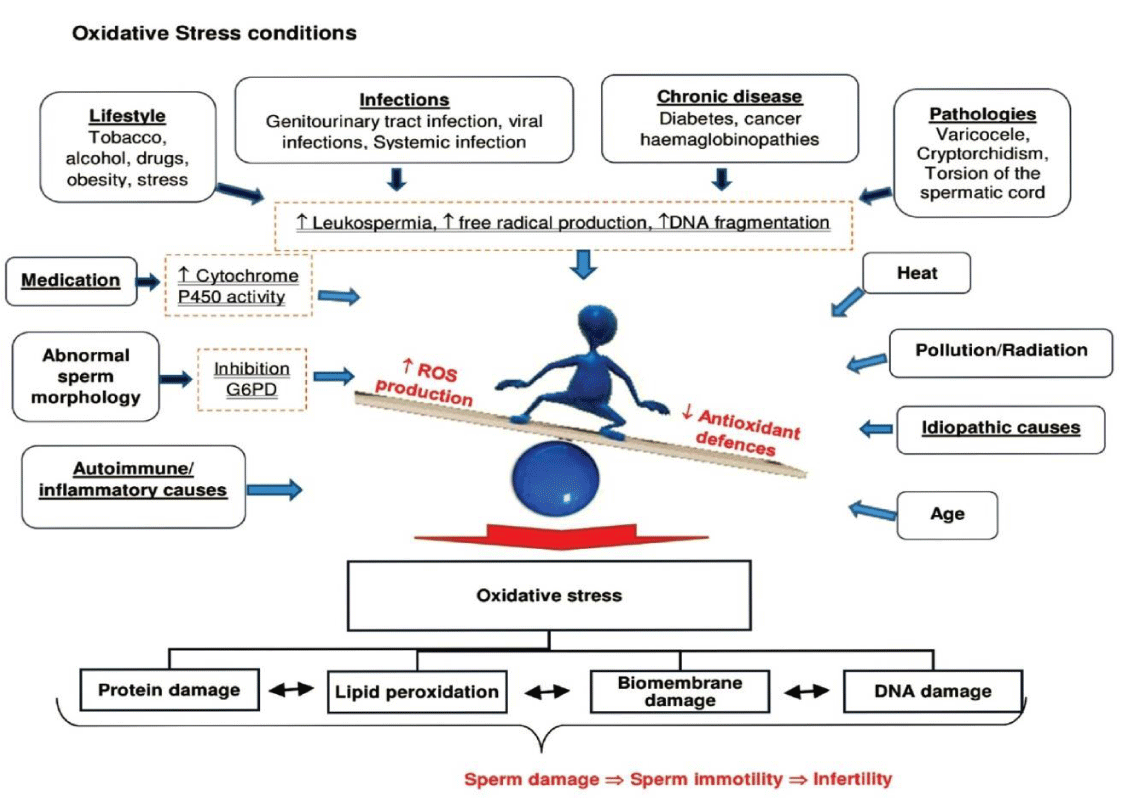
Oxidative stress is the root for many disease conditions such as Infertility. It is caused due to an imbalance between pro-oxidants and antioxidants [1]. This imbalance is created due to enhanced levels of ROS (Reactive Oxygen Species) and / or RNS (Reactive Nitrogen Species) in plasma and blood of patients. In addition, there is often a decreased level of antioxidant mechanism, thereby deteriorating the balance ratio.
As oxidative stress is one of the major causes for infertility, excessive generation of ROS causes an environment unsuitable for normal female physiological reactions [1]. This leads to reproductive diseases such as PCOS, Endometriosis and unexplained infertility in women [2]. Pregnancy complications such as miscarriage, recurrent respiratory loss (RPL), preeclampsia and intrauterine growth restriction, IUGR, are all linked to oxidative stress [3].
Not only maternal, but paternal reproductive disease conditions are also due to oxidative stress. In this review, some of them such as Impairment of Spermatozoa, Sperm Dysfunction, Sperm DNA Fragmentation (SDF), are discussed briefly.
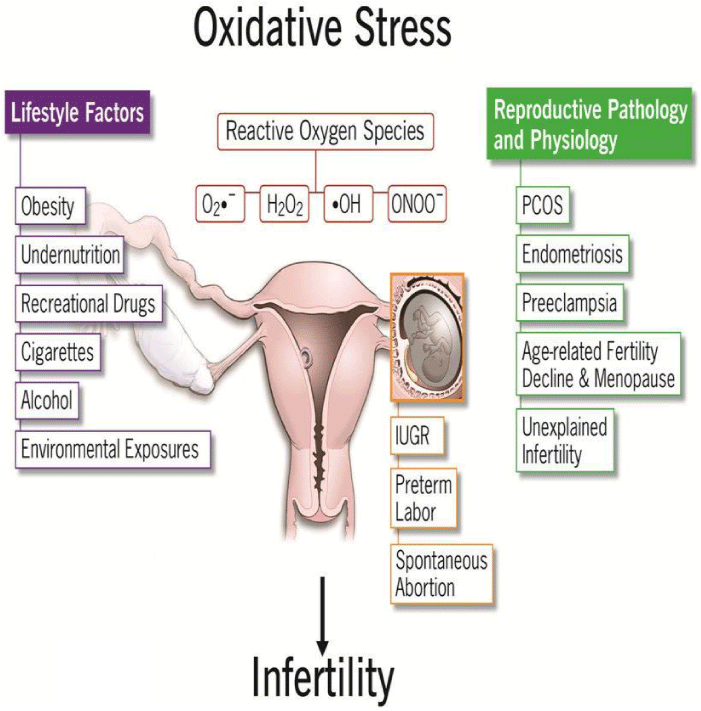
Various factors are also responsible for infertility, such as obesity, malnutrition, life style factors – smoking, alcohol and recreational drugs [4], environmental and occupational exposures – pesticides and endocrine disrupting chemicals, EDCs [5-7]. These factors are linked to oxidative stress.
This problem of infertility due to oxidative stress or other reasons, can be solved by Antioxidant supplementation such as glutathione and/ or Assisted Reproductive Techniques, ART [2], finally to bring a smile on the couple’s face. Measurement of oxidation-reduction potential by MiOXSYS® system, is yet another approach to solve the problem of oxidative stress [8].
Major complications due to oxidative stress, pertaining to infertility:
1. Male Infertility - i) Impairment of Spermatozoa
ii) Sperm Dysfunction
iii) Sperm DNA Fragmentation (SDF)
2. Female Infertility – i) Polycystic Ovary Syndrome (PCOS)
ii) Endometriosis
3. Pregnancy complications –i) The Placenta
ii) Miscarriage
iii) Recurrent pregnancy loss
iv) Preeclampsia
Male Infertility
i) Impairment of Spermatozoa in humans: Impaired sperms are one of the major causes of infertility. It has been documented that the nucleohistone compartment of sperms which contains histone-bound DNA sequences like telomeres and promoters of genes for embryonic development, are often at a high risk of oxidative stress, leading to infertility [8,9]. Also, the relative decrease in protamine 2 levels, in the ratio of protamine1: protamine2, at the level of m-RNA and protein, also results in infertility. Again, gene mutations encoding protamines lead to structural changes in sperm chromatin structure, thereby leading to infertility [10,11].
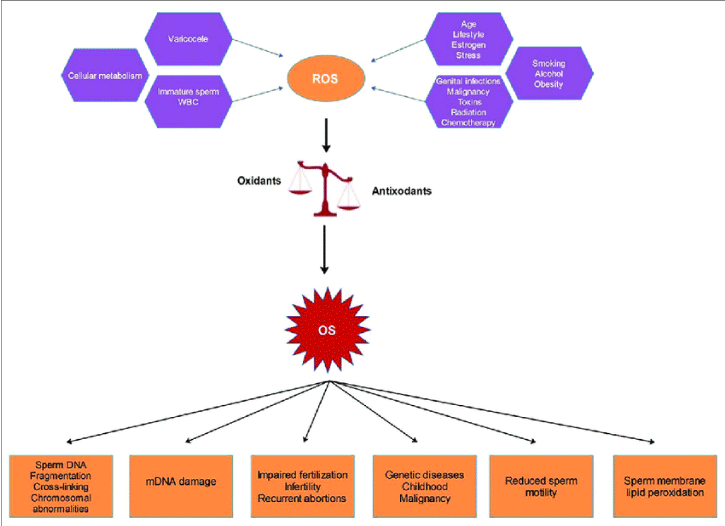
ii) Sperm dysfunction: The mechanism of sperm dysfunction is related to the oxidative stress, which actually causes distortion of the sperm DNA integrity, by damaging proteins and lipids present in sperm cell plasma membrane. This has a negative effect on sperm cell membrane fluidity and permeability, leading to infertility [12]. Also, it has been established that sperm motility gets reduced as ROS becomes directly proportional to the level of lipid peroxidation [13]. The sperm dysfunction is also related to the lipid peroxidation cascade activation, initiated by ROS-mediated oxidation of lipids in sperm cell membrane. Figure 1, explains the same.
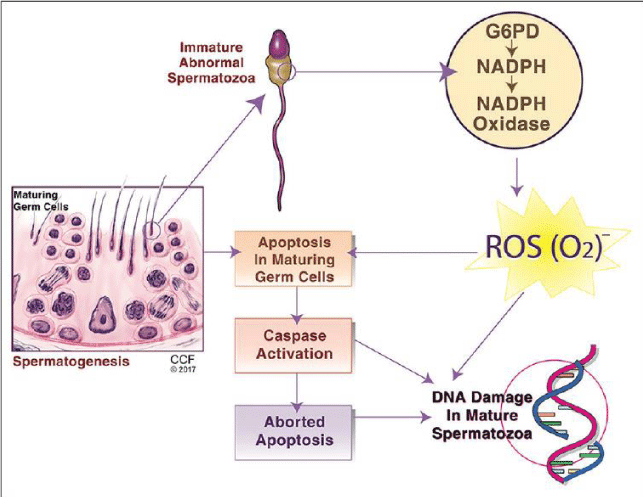
iii) Sperm DNA Fragmentation (SDF): According to the researchers, SDF is mediated by free-radicals [14,15]. Again, the mechanisms underlying SDF include single-strand and double-strand breaks, DNA fragmentation, the introduction of abasic sites, purine, pyrimidine and deoxyribose modifications and DNA crosslinking, which can result in arrest or induction of gene transcription, induction of signal transduction pathways, accelerated telomeric DNA attrition, replication errors, genomic instability and GC to TA transversions [16-18]. As such mechanisms are also known to be the causes of carcinogenesis, these may explain a link between infertility and cancer. Also, male infertility is closely associated with ROS-induced DNA damage, which in turn accelerates the germ-cell apoptosis process, leading to decline in sperm count [19]. Figure 2 explains the same.
Female Infertility
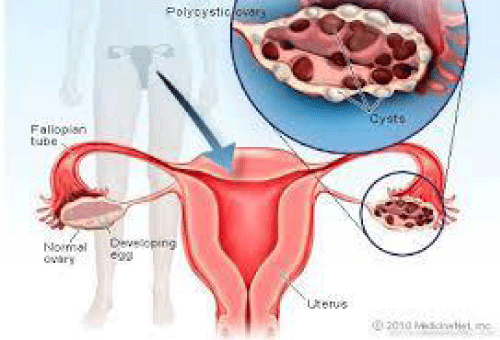
i) Polycystic Ovary Syndrome (PCOS): The word “polycystic” means “many cysts. PCOS is said to develop when within the ovaries, numerous minute fluid-filled sacs appear. Each of these sacs is a follicle which holds an immature egg. These eggs do not develop to complete the ovulation process. Again, due to this lack of ovulation, there is a change in the hormonal levels of the female body. For eg. estrogen and progesterone levels get reduced while androgen levels are higher. The menstrual cycle in the females therefore, gets disturbed due to hyperandrogenism. So, women with fewer periods than normal, often lead to PCOS. Figure 3 explains the same.
Several disease conditions arise due to pathogenesis of PCOS: Hyperinsulinemia, reproductive aberration, hyperandrogenism, oligomenorrhea, amenorrhea, obesity complications, acanthosis nigricans, insulin resistance and genetic factor complications [20]. 6-10% of premenopausal women suffer from PCOS [21].
Oxidative stress (OS) and PCOS
Oxidative stress leads to PCOS. It is well established, that ROS (Reactive Oxygen Species) is more in PCOS patients, especially for insulin resistance patients [22]. Also, a decrease in GSH content and GSH to GSSG ratio in leukocytes suggests a deterioration of mitochondrial function and an increase in oxidative stress leading to PCOS. As Protein carbonyl (PC) is an important biomarker of oxidative stress, it is observed that PC content is higher in PCOS patients compared to control. Further, PC content is known to have a positive correlation with fasting insulin, suggesting a strong association between insulin resistance and protein oxidation in PCOS. Malonal dialdehyde or MDA (produced during decomposition of polyunsaturated fatty acids) is one of the biomarkers of OS. It is seen that blood MDA levels are significantly (P= 0.01) higher in young, lean, non-IR PCOS patients, in comparison to the control [23]. Again, SOD (Superoxide dismutase) levels are higher in PCOS patients, as compared to control. As IL-6 (Interleukin-6) and TNF-α (Tumor necrosis factor alpha) are pro-inflammatory markers of oxidative stress, it is well established that IL-6 is more in PCOS patients and shoots up in the case of PCOS-IR patients [22]. TNF-α, on the other hand is higher in both PCOS-IR as well as PCOS-non IR patients.
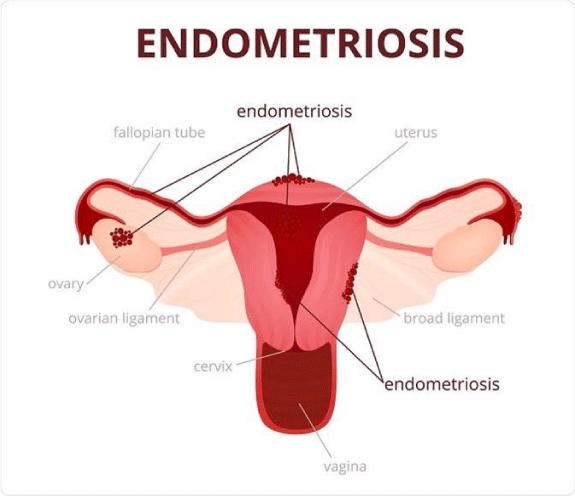
ii) Endometriosis: Endometriosis occurs when the tissue that forms the uterine lining (the lining of the womb) is present on various organs within the body. Endometriosis is usually found in the lower abdomen, or pelvis, but can also appear elsewhere in the body. Endometriosis is explained by Figure 4.
Women with endometriosis often suffer from lower abdominal pain, pain with periods, or pain with sexual intercourse, and may report having a hard time getting pregnant. However, some women with Endometriosis, may be asymptomatic.
Endometriosis and Fertility
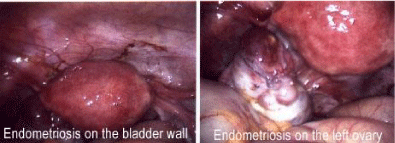
Between 20 and 40% of women with infertility, normally suffer from endometriosis. Endometriosis seems to impair fertility in 2 ways: firstly, by causing deformation in the fallopian tubes so that they are unable to pick up the egg after ovulation, and secondly, by causing inflammations that in turn causes impairment of the functioning of the ovary, egg, fallopian tubes or uterus. Figure 5 shows the endometriosis tissue on the bladder wall and left ovary.
Pregnancy complications
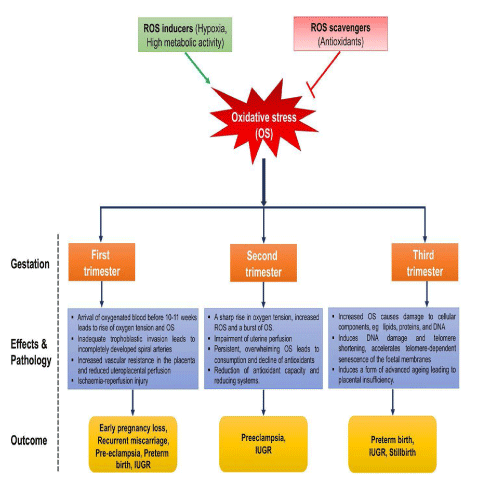
i) The Placenta: In cases of incomplete placentation, oxidative stress occurs at an excessive level and leads to an elevated release of placental debris and vesicles into the maternal circulation. These extracellular vesicles carry active molecules (proteins of microRNA) generated by stressed placental cells. Once in the blood, they meet maternal endothelial cells and potentially transfer their contents, leading to transcriptome alterations and inflammation. Eventually, the endothelium of maternal organs is affected along with the deterioration of synciotrophoblast [24]. Figure 6 explains the effect of oxidative stress on placental function and pathological events in pregnancy.
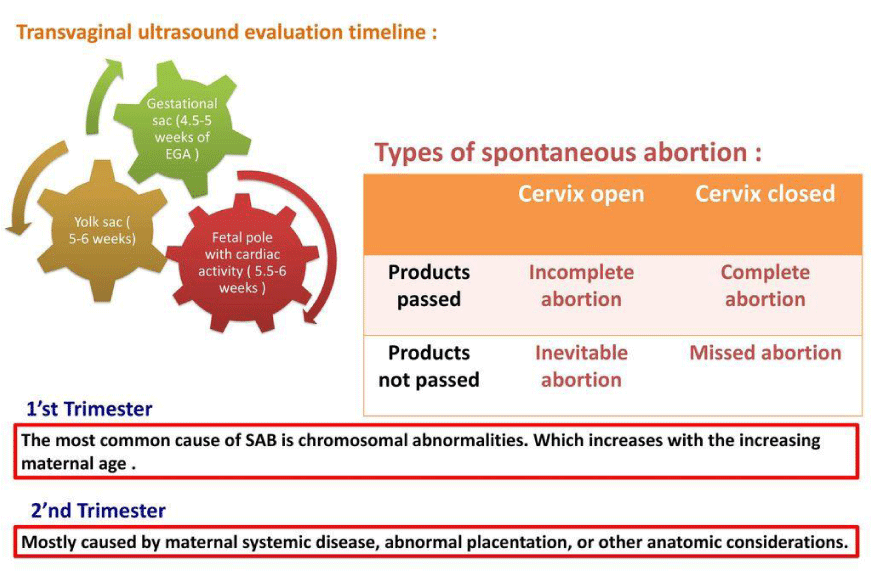
ii) Miscarriage or Spontaneous Abortion: Miscarriage or spontaneous abortion is the unintentional termination of pregnancy when the fetal weight is < 500g. Placental oxidative stress is one of the major cause of miscarriage. Due to premature intraplacental circulation at 8 to 9 weeks of pregnancy, instead of 10-12 weeks of gestation, high oxidative stress is set up [25,26] which distorts the syncytiotrophoblast layer of the placenta, thereby causing impairment of the placenta [27]. Apoptosis of the placental tissues may also occur due to oxidative stress- induced inflammatory processes. Hence, due to this degeneration of the placenta, miscarriage often occurs [2]. Figure 7 depicts the types of spontaneous abortion.
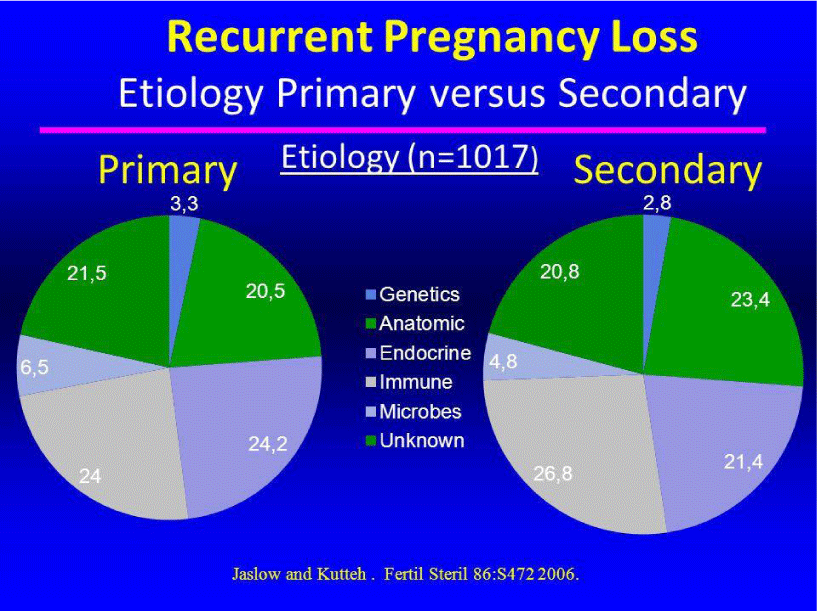
iii) Recurrent Pregnancy Loss (RPL): Recurrent pregnancy loss refers to more than three consecutive pregnancy losses. The reason behind this situation is the enhancement of Natural Killer cells (NK cells) in placental blood circulation, thereby increasing pre-implantation angiogenesis. This in turn causes premature intra-placental maternal circulation, leading to very high oxidative stress [28]. It has been well established, that in patients with RPL, there is an enhancement of lipid peroxides and GSH in plasma [29], in addition to the reduction in levels of vitamin E and ß-carotene, indicating en elevated level of oxidative stress [30]. Another study, reveals that there is significantly decreased levels of antioxidant enzymes like GPx, SOD and catalase, in addition to increased MDA levels, in patients with RPL, again indicating augmented oxidative stress [31]. Figure 8, explains the etiology of Recurrent Pregnancy loss, that is, Primary versus Secondary.
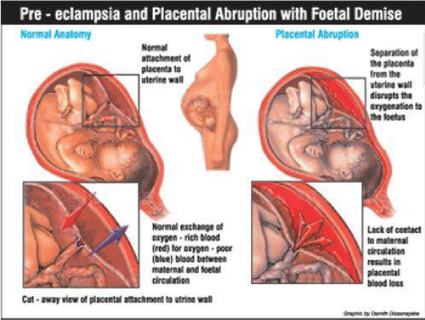
iv) Preeclampsia: Preeclampsia refers to a complex multisystem disorder that is characterized by high blood pressure pregnancy complication. This is a leading cause of maternal and fetal morbidity, accounting to 3% to 14% of pregnancies worldwide [32, 33]. In this context, Placental ischemia /hypoxia plays a very important role in augmenting the oxidative stress, which in turn leads to endothelial cell dysfunction [33, 34] and systemic vasoconstriction [35]. Also, it is seen that the oxidative stress has contributed to the apoptosis of villous trophoblasts, in patients with Preeclampsia [2]. Figure 9 explains Preeclampsia and Placental Abruption, diagrammatically.
Factors governing Infertility, associated with Oxidative Stress:
1. Age
2. Body weight – i) Obesity
iii) Underweight
3. Lifestyle factors – i) Cigarette
ii) Alcohol usage
iii) Intake of Recreational drugs
iii) Intake of Recreational drugs
4. Environmental and occupational exposures –
i) Pesticides
ii) Endocrine Disrupting Chemicals (EDCs)
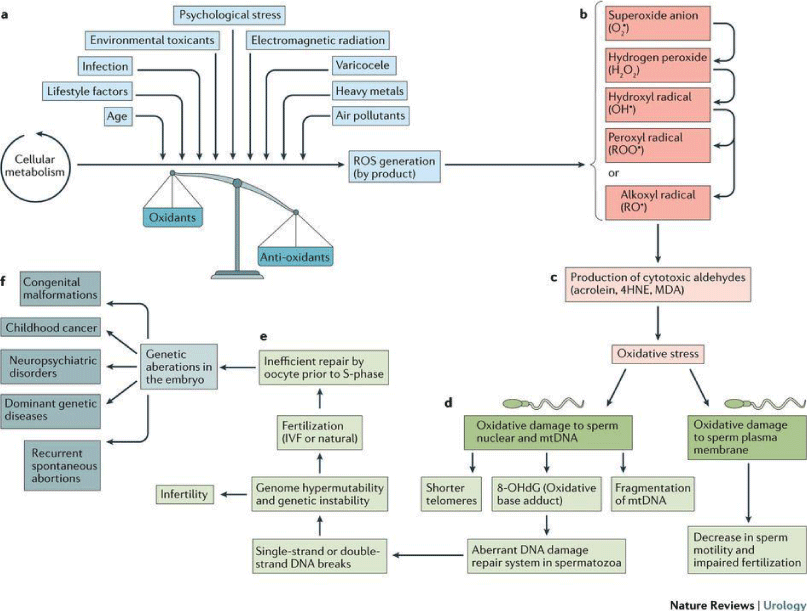
Figures 10,11, explain the various factors leading to female and male infertility.
1. Age: Age is a vital factor for infertility, as fertility declines with advancing mother’s age. Paternal age is also important in terms of fertility, as retrogression in gamete quality, increased levels of oxidative DNA damage (ODD) and decline in semen quality. Hence, sperm DNA is often exposed to oxidative stress because of advancement in paternal age [9,36].
2. Body weight
i) Obesity: The pathogenesis of obesity is closely associated to the excessive generation of ROS, thereby setting up of Oxidative stress [32]. Hence, overweight can complicate pregnancy issues, both in terms of maternal as well as fetal health. Also, dysfunctional hormonal system and irregular menses, are known to be closely associated with overweight or obesity [38].
ii) Underweight: Malnourishment in reproductive females is associated to deterioration of endothelium- dependent vasodilation, triggering oxidative stress [39].
3. Lifestyle Factors
i) Cigarette smoking: It has been well established that infertility, pregnancy complications, damage to the developing embryo, higher rates of fetal loss, decreased fetal growth, preterm birth and miscarriage, are all the results of maternal smoking [2,40,41]. As cigarette smoking is composed of many toxic chemicals and pro-oxidants, it triggers the production of ROS, setting up oxidative stress in follicular microenvironment [42].
ii) Alcohol usage: Alcohol consumption gives rise to the production of metabolites like acetyl and methyl radicals, responsible for ROS generation. Regular alcohol consumption, triggers lipid peroxidation and lowers SOD antioxidant activity along with GSH levels, thereby elevating the level of ROS in plasma of pregnant mother. Hence, maternal alcohol usage can thus result in Intrauterine Growth Restriction (IUGR), low birth weight, increased risk of congenital disorder, early pregnancy loss and spontaneous abortion (miscarriage) [2,4].
iii) Recreational Drug usage: The active constituent of Marijuana (Recreational drug) that is Cannabinoids, produce free radicals which can alter central as well as peripheral nervous system functioning [43]. Another important constituent of the drug which causes psychological impacts in smokers is called delta-9-tetrahydrocannabinol (THC). This THC is known to induce breakage in DNA strands (Chan et al. 1998), being associated with ROS generation [44-46].
Cocaine, another important addictive drug, triggers lipid peroxidation, through several metabolites, thereby producing oxidative stress and lipid peroxyl radicals [47]. While formaldehyde (oxidative metabolites of cocaine) generates ROS, Norcocaine another oxidative metabolite of cocaine causes oxidative stress by depleting GSH stores [4]. Also, ROS induces apoptosis [48,49].
4. Environmental and Occupational exposures
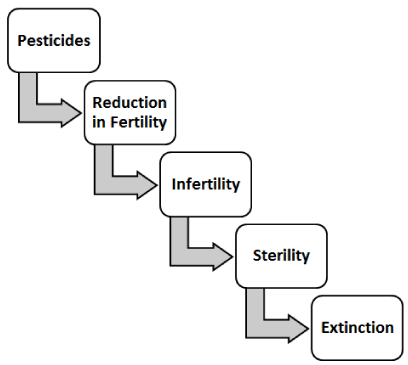
i) Pesticides: It has been well documented that the spouses of agricultural male workers who are in direct contact with pesticide chemicals like DDT (Organochlorine pesticides), are victims of miscarriage or spontaneous abortion [50].
Again, polychlorinated biphenyls or PCBs (pesticide) are known to enhance free radical generation by setting up oxidative stress, through induction of endothelial dysfunction [51] and cell membrane destruction [2]. Interestingly, PCB exposure causes suppression of Vitamin E levels [51].
Organophosphate pesticides on the other hand, causes depletion of GSH along with increase in ROS, triggering oxidative stress [52-54]. Figure 12, explains the role of pesticides in infertility.
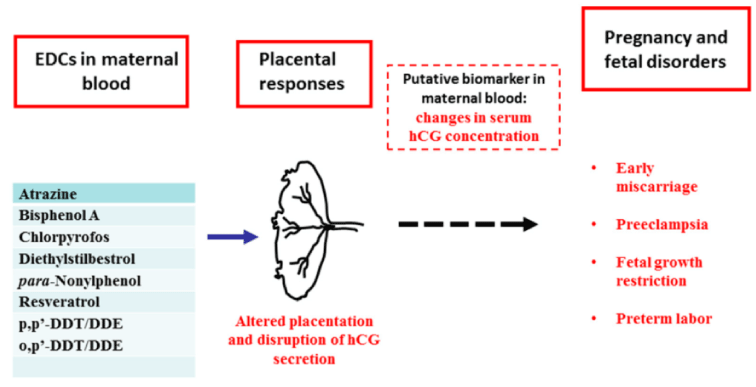
ii) Endocrine Disrupting Chemicals (EDCs): Endocrine Disrupting Chemicals (EDCs) like the phthalates acts as an anti-androgenic compound, deteriorating the male reproductive system and is associated with the setting up of the oxidative stress [55]. Also, it has been documented, that there is an association between urinary phthalate metabolites and polyvinyl chloride workers [55]. After taking into consideration, the age, smoking status and coffee consumption, it has been observed that the urinary phthalate metabolites causes sperm apoptosis and ROS generation. Limited evidence suggests that BPA (Bisphenol A) sets up oxidative stress, which in turn impairs the male reproductive capacity. Figure 13, explains the role of EDCs in infertility.
Therapeutic solutions to Infertility
i) Antioxidant Supplementation
ii) Assisted Reproductive Techniques
i) Antioxidant Supplementation
As oxidative stress is one of the main causes of infertility, antioxidant supplementation is required to scavenge the free radicals, thereby creating a balance between ROS and antioxidants. Antioxidants such as vitamin C, vitamin E, selenium, zinc and glutathione are important for male infertility [56,57]. Table 1, depicts the antioxidants, their characteristics and effects on semen parameters.
Glutathione – the principal antioxidant for male infertility
Glutathione prevents male infertility by scavenging lipid peroxides in the plasma membrane of spermatozoa, thereby reducing the oxidative stress. It also scavenges hydrogen peroxide, thereby arresting lipid peroxidation and reducing oxidative stress [20].
Glutathione – the principal antioxidant for female infertility
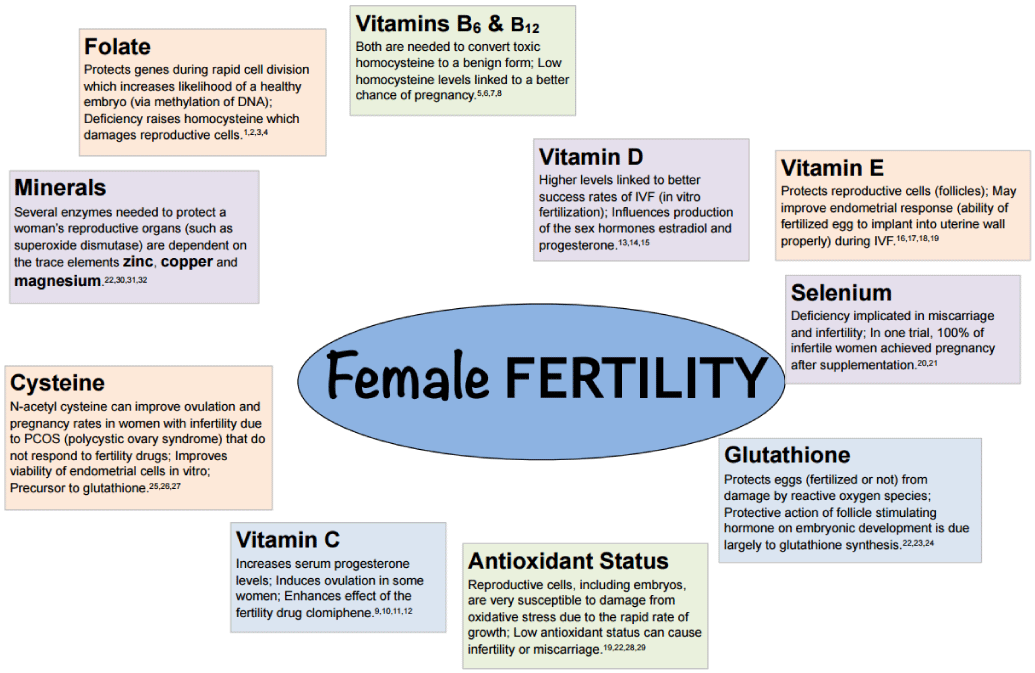
During folliculogenesis, Glutathione shields eggs from oxidative stress [20]. Researches, in this context have thus proved that oocytes with elevated levels of intracellular glutathione produce healthier and stronger embryos [58]. Figure 14, explains the antioxidant supplementation important for female fertility.
Glutathione – impact on autoimmune issues
Glutathione is indeed a boon for those with immunological miscarriages or if the body is rejecting one’s mate’s sperm [20].
ii) Assisted Reproductive techniques
Assisted Reproductive techniques are the treatments of choice for the infertile men or women. In cases of endometriosis, tubal factor infertility, male factor infertility and unexplained infertility, these techniques can bring a new born baby to the affected couple [59].
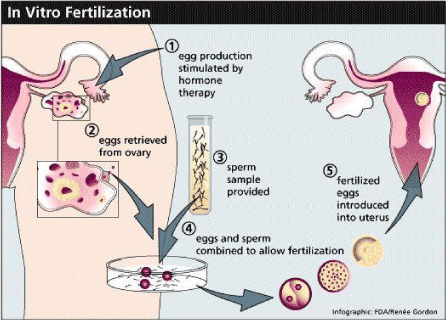
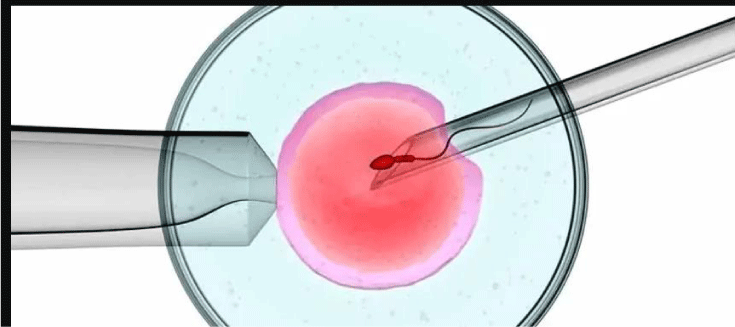
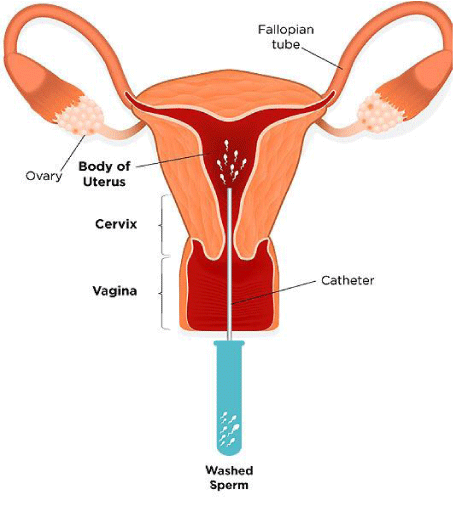
These techniques include intrauterine insemination, IVF and intracytoplasmic sperm injection (ICSI). The same have been explained by Figures 15-17.
Conclusion
As oxidative stress is one of the major causes of infertility (Figure 18), couples especially must include antioxidants in diet, refrain themselves from bad habits such as smoking, alcohol, recreational drugs and perform yoga and meditation daily, to ensure a happy family life with children. Further research work on Endocrine Disrupting Chemicals (EDCs) and endometriosis [60] need to be carried out, in order to elucidate the path of human fertility. Advancement in science and technology have also gifted techniques such as Assisted Reproductive Techniques (ART), which aims to bring a smile on the couple’s face.
- Al-Gubory KH, Fowler PA, Garrel C (2010) The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol 42: 1634-1650. Link: https://bit.ly/336SGbQ
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S (2012) The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 10:49. Link: https://bit.ly/31bnJBw
- Webster RP, Roberts VH, Myatt L (2008) Protein nitration in placenta – functional significance. Placenta 29: 985-994. Link: https://bit.ly/2OxsMug
- Kovacic P (2005) Unifying mechanism for addiction and toxicity of abused drugs with application to dopamine and glutamate mediators: electron transfer and reactive oxygen species. Med Hypotheses 65: 90-96. Link: https://bit.ly/2OwNmuO
- Hennig B, Hammock BD, Slim R, Toborek M, Saraswathi V, et al. (2002) PCB-induced oxidative stress in endothelial cells: modulation by nutrients. Int J Hyg Environ Health 205: 95-102. Link: https://bit.ly/2STrAjq
- Jirsova S, Masata J, Jech L, Zvarova J (2010) Effect of polychlorinated biphenyls (PCBs) and 1,1,1-trichloro-2,2,-bis (4-chlorophenyl)-ethane (DDT) in follicular fluid on the results of in vitro fertilization-embryo transfer (IVF-ET) programs. Fertil Steril 93: 1831-1836. Link: https://bit.ly/2ZjOcMu
- Samarawickrema N, Pathmeswaran A, Wickremasinghe R, Peiris-John R, Karunaratna M, et al. (2008) Fetal effects of environmental exposure of pregnant women to organophosphorus compounds in a rural farming community in Sri Lanka. Clin Toxicol (Phila) 46:489-495. Link: http://bit.ly/2OxtXda
- Martins AD, Agarwal A (2019) Oxidation-Reduction potential : a new biomarker of male infertility. Panminerva Medi 61: 108-17. Link: http://bit.ly/2LUoprg
- Dada R, Bisht S (2017) Oxidative Stress and Male Infertility. Link: http://bit.ly/2OvMtCV
- Aoki VW, Christensen GL, Atkins JF, Carrell DT (2006) Identification of novel polymorphisms in the nuclear protein genes and their relationship with human sperm protamine deficiency and severe male infertility. Fertil Steril 86: 1416-1422. Link: http://bit.ly/2LTbupA
- Jiang W, Sun H, Zhang J, Zhou Q, Wu Q, et al. (2015) Polymorphisms in protamine 1 and protamine 2 predict the risk of male infertility: a meta-analysis. Sci Rep 5: 15300. Link: https://go.nature.com/2LSRi79
- Aitken RJ (2013) Human spermatozoa: revelations on the road to conception. F1000 Prime Rep 5: 39. Link: http://bit.ly/33dZowG
- Gomez E, Irvine D, Aitken R (1998) Evaluation of a spectrophotometric assay for the measurement of malondialdehyde and 4‑hydroxy-alkenals in human spermatozoa: relationships with semen quality and sperm function. Int J Androl 21: 81-94. Link: http://bit.ly/2yvH0kJ
- Dorostghoal M, Kazeminejad S, Shahbazian N, Pourmehdi M, Jabbari A (2017) Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia 49. Link: http://bit.ly/2YELYdo
- Iommiello VM, Albani E, Di Rosa A, Marras A, Menduni F, et al. (2015) Ejaculate oxidative stress is related with sperm DNA fragmentation and round cells. International journal of endocrinology 321901. Link: http://bit.ly/2ZmNKwY
- Bauer NC, Corbett AH, Doetsch PW (2015) The current state of eukaryotic DNA base damage and repair. Nucleic Acids Res 43: 10083-10101. Link: http://bit.ly/2MuziQ3
- Hosen MB, Islam MR, Begum F, Kabir Y, Howlader MZH (2015) Oxidative stress induced sperm DNA damage, a possible reason for male infertility. Iran J Reprod Med 13: 525-532. Link: http://bit.ly/2Zqj6To
- Li Z, Yang J and Huang H (2006) Oxidative stress induces H2AX phosphorylation in human spermatozoa. FEBS Lett 580: 6161-6168. Link: http://bit.ly/2ZoS0w2
- Aitken RJ, Krausz C (2001) Oxidative stress, DNA damage and the Y chromosome. Reproduction 122: 497-506. Link: http://bit.ly/2Mxak2f
- Adeleke O (2018) Review on the role of glutathione on oxidative stress and infertility. Link: http://bit.ly/312d8IO
- Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, et al. (2000) A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 85: 2434-8. Link: http://bit.ly/2ytO80W
- Victor VM., Rocha M, Bañuls C, Alvarez A, de Pablo C, et al. (2011) Induction of Oxidative Stress and Human Leukocyte/ Endothelial Cell Interactions in Polycystic Ovary Syndrome Patients with Insulin Resistance. J Clin Endocrinol Metab 96: 3115-3122. Link: http://bit.ly/2SURHXn
- Lee JY, Baw C-K, Gupta S, Aziz N, Agarwal A (2010) Role of Oxidative Stress in Polycystic Ovary Syndrome. Current Women’s Health Reviews 6: 96-107. Link: http://bit.ly/2YjUvmL
- Aouache R, Biquard L, Vaiman D, Miralles F (2018) Oxidative Stress in Preeclampsia and Placental Diseases Int J Mol Sci 19: pii: E1496. Link: http://bit.ly/2YD9Ied
- Watson AL, Skepper JN, Jauniaux E, Burton GJ (1998) Changes in concentration, localization and activity of catalase within the human placenta during early gestation. Placenta 19: 27-34. Link: http://bit.ly/3301YWN
- Jauniaux E, Gulbis B, Burton GJ (2003) Physiological implications of the maternofetal oxygen gradient in human early pregnancy. Reprod Biomed Online 7: 250-253. Link: http://bit.ly/2LTfUNc
- Gupta S, Agarwal A, Banerjee J, Alvarez JG (2007) The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. Obstet Gynecol Surv 62: 335-347. Link: http://bit.ly/2yudj3s
- Quenby S, Nik H, Innes B, Lash G, Turner M, et al. (2009) Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum Reprod 24: 45-54. Link: http://bit.ly/2KePuSJ
- Simsek M, Naziroglu M, Simsek H, Cay M, Aksakal M, et al. (1998) Blood plasma levels of lipoperoxides, glutathione peroxidase, beta carotene, vitamin A and E in women with habitual abortion. Cell Biochem Funct 16: 227-231. Link: http://bit.ly/2GFGbKz
- Miller H, Wilson R, Jenkins C, MacLean MA, Roberts J, et al. (2000) Glutathione levels and miscarriage. Fertil Steril 74: 1257-1258. Link: http://bit.ly/2SUppfL
- El-Far M, El-Sayed IH, El-Motwally Ael G, Hashem IA, Bakry N (2007) Tumor necrosis factor-alpha and oxidant status are essential participating factors in unexplained recurrent spontaneous abortions. Clin Chem Lab Med 45: 879-883. Link: http://bit.ly/2YICD4F
- Gupta S, Aziz N, Sekhon L, Agarwal R, Mansour G, et al. (2009) Lipid peroxidation and antioxidant status in preeclampsia: a systematic review. Obstet Gynecol Surv 64: 750-759. Link: http://bit.ly/2T2PFog
- Reslan OM, Khalil RA (2010) Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc Hematol Agents Med Chem 8: 204-226. Link: http://bit.ly/315SQy5
- Myatt L, Cui X (2004) Oxidative stress in the placenta. Histochem Cell Biol 122: 369-382. Link: http://bit.ly/2Yx6psJ
- Khalil RA, Granger JP (2002) Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol 283: R29-R45. Link: http://bit.ly/2YzH39R
- Crow JF (2000) The origins, patterns and implications of human spontaneous mutation Nat Rev Genet 1: 40-47. Link: http://bit.ly/2Ykwfks
- Bloomer RJ, Fisher-Wellman KH (2009) Systemic oxidative stress is increased to a greater degree in young, obese women following consumption of a high fat meal. Oxid Med Cell Longev 2: 19-25. Link: http://bit.ly/2ZoFfSd
- Herrero-Mercado M, Waliszewski SM, Caba M, Martinez-Valenzuela C, Gomez Arroyo S, et al. (2011) Organochlorine pesticide gradient levels among maternal adipose tissue, maternal blood serum and umbilical blood serum. Bull Environ Contam Toxicol 86: 289-293. Link: http://bit.ly/2SZ96hF
- Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, et al. (2003) Low body mass index is a risk factor for impaired endothelium-dependent vasodilation in humans: role of nitric oxide and oxidative stress. J Am Coll Cardiol 42: 256-263. Link: http://bit.ly/334zb3u
- Huang J, Okuka M, McLean M, Keefe DL, Liu L (2009) Effects of cigarette smoke on fertilization and embryo development in vivo. Fertil Steril 92:1456-1465. Link: http://bit.ly/2GFJsJR
- Kumar S (2011) Occupational, environmental and lifestyle factors associated with spontaneous abortion. Reprod Sci 18: 915-930. Link: http://bit.ly/2MxwKRg
- Tiboni GM, Bucciarelli T, Giampietro F, Sulpizio M, Di Ilio C (2004) Influence of cigarette smoking on vitamin E, vitamin A, beta-carotene and lycopene concentrations in human pre-ovulatory follicular fluid. Int J Immunopathol Pharmacol 17: 389-393. Link: http://bit.ly/2KeR5rH
- Chan GC, Hinds TR, Impey S, Storm DR (1998) Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol. J Neurosci 18: 5322-5332. Link: http://bit.ly/2GEkdHZ
- Kovacic P, Jacintho JD (2001) Reproductive toxins: pervasive theme of oxidative stress and electron transfer. Curr Med Chem 8: 863-892. Link: http://bit.ly/2LQCaHn
- Kovacic P, Sacman A, Wu-Weis M (2002) Nephrotoxins: widespread role of oxidative stress and electron transfer. Curr Med Chem 9: 823-847. Link: http://bit.ly/2SWPQkG
- Kovacic P, Jacintho JD (2001) Mechanisms of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem 8: 773-796. Link: http://bit.ly/2Ykhuyb
- Lloyd RV, Shuster L, Mason RP (1993) Reexamination of the microsomal transformation of N-hydroxynorcocaine to norcocaine nitroxide. Mol Pharmacol 43: 645-648. Link: http://bit.ly/2Ydc5sy
- Sharan N, Chong VZ, Nair VD, Mishra RK, Hayes RJ, et al. (2003) Cocaine treatment increases expression of a 40 kDa catecholamine-regulated protein in discrete brain regions. Synapse 47: 33-44. Link: http://bit.ly/2MzzLjR
- Zhang L, Xiao Y, He J (1999) Cocaine and apoptosis in myocardial cells. Anat Rec 257: 208-216. Link: http://bit.ly/2YyzJiJ
- Petrelli G, Figa-Talamanca I, Lauria L, Mantovani A (2003) Spontaneous abortion in spouses of greenhouse workers exposed to pesticides. Environ Health Prev Med 8: 77-81. Link: http://bit.ly/2ZnQOcb
- Hennig B, Slim R, Toborek M, Robertson LW (1999) Linoleic acid amplifies polychlorinated biphenyl-mediated dysfunction of endothelial cells. J Biochem Mol Toxicol 13: 83-91. Link: http://bit.ly/2KgOSMn
- Banerjee BD, Seth V, Ahmed RS (2001) Pesticide-induced oxidative stress: perspectives and trends. Rev Environ Health 16: 1-40. Link: http://bit.ly/2GDqgwe
- Delescluse C, Ledirac N, Li R, Piechocki MP, Hines RN, et al. (2001) Induction of cytochrome P450 1A1 gene expression, oxidative stress, and genotoxicity by carbaryl and thiabendazole in transfected human HepG2 and lymphoblastoid cells. Biochem Pharmacol 61: 399-407. Link: http://bit.ly/2YD2lau
- Halliwell B (2002) Effect of diet on cancer development: is oxidative DNA damage a biomarker? Free Radic Biol Med 32: 968-974. Link: http://bit.ly/2OHguQ2
- Jeng HA (2014) Exposure to endocrine disrupting chemicals and male reproductive health. Front Public Health 2: 1-12. Link: http://bit.ly/2ytqRMx
- Gharagozloo P, Gutiérrez-Adán A, Champroux A, Noblanc A, Kocer A, et al. (2016) A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: promising preclinical evidence from animal models. Hum Reprod 31: 252-262. Link: http://bit.ly/2ZlcLsA
- Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H et al. (2003) Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl 49: 83-94. Link: http://bit.ly/2YA7wb8
- Mukherjee A, Malik H, Saha AP, Dubey A, Singhal DK, et al. (2014) Resveratrol treatment during goat oocytes maturation enhances developmental competence of parthenogenetic and hand-made cloned blastocysts by modulating intracellular glutathione level and embryonic gene expression. J Assist Reprod Genet 31: 229-39. Link: http://bit.ly/2M0yFOT
- Gupta S, Sekhon L, Agarwal A (2010) The role of oxidative stress and antioxidants in assisted reproduction. Curr Wom Health Rev 6: 227-238. Link: http://bit.ly/3338U5F
- Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, et al. (2017) Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid Med Cell Longev. Link: http://bit.ly/2Yk9xJd
- Pereira R, Sá R, Barros A and Sousa M (2017) Causes that lead to oxidative stress condition, digital photograph. Asian journal of andrology. Link: http://bit.ly/332yEPr
- Majzoub A and Agarwal A (2017) Mechanisms of sperm DNA fragmentation in human spermatozoa, digital photograph. Indian J of Urology. Link: http://bit.ly/319Jmlp
- Stöppler MC (2018) Polycystic Ovary Syndrome (PCOS), digital photograph. emedicine health. Link: http://bit.ly/2YgVtQO
- Endometriosis, digital photograph (2017) Ovary pain: All you need to know. Link: http://bit.ly/2Kn2ZQc
- Liu CY (2011) Endometriosis on the bladder wall and Endometriosis in the left ovary, digital photographs. What is Endometriosis ? Link: http://bit.ly/2MCQ3sf
- Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, et al. (2017) Effect of oxidative stress on placental function and pathological events in pregnancy, digital photograph. Am J Reprod Immunol. Link: http://bit.ly/2yAbZfe
- Types of Spontaneous Abortion, digital photograph (2019) Obstetrics & Gynecology, Spontaneous Abortion. Link: http://bit.ly/2OwTPGf
- Schnorr JA Recurrent Pregnancy Loss (2019) Etiology Primary versus Secondary, digital photograph. Evaluation of Recurrent Pregnancy Loss, An Evidence Based Approach. Link:http://bit.ly/2Yh1Wv0
- (2019)Pre-eclampsia and Placental Abruption with Foetal Demise, digital photograph. the Doe Report. Link: http://bit.ly/335xl2n
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A and Gupta S (2012) Factors contributing to the development of oxidative stress and their impacts on female reproduction, digital photograph. Reproductive Biology and Endocrinology. Link: http://bit.ly/3384cDs
- Bisht S, Faiq M, Tolahunase M and Dada R (2017) Various factors contributing to oxidative stress and bringing about male infertility, digital photograph. Nature Reviews, Urology. Link: https://go.nature.com/2SYhluk
- Sharma RK, Goyal AK (2014) Role of pesticides in infertility, digital photograph. Int J Pharm Pharm Sci. Link: http://bit.ly/2YA0nDB
- Paulesu L, Rao ChV, Ietta F, Pietropolli A and Ticconi C (2018) Role of Endocrine Disrupting Chemicals (EDCs) in infertility, digital photograph. International Journal of Molecular Sciences. Link: http://bit.ly/2KakHHK
- Bisht S, Faiq, M, Tolahunase M and Dada R (2017) Antioxidants, their characteristics and effects on semen parameters, Table no. 3. Nature Reviews, Urology. Link:https://go.nature.com/2SYhluk
- (2018) Antioxidant suplementation important for female infertility, digital photograph. Boosting fertility with nutrition. Link: http://bit.ly/2GH6dxc
- In Vitro Fertilization (2019) Digital photograph. In Vitro Fertilization, ivf process and Embryo Transfer (IVF - ET). Link: http://bit.ly/2KiXmmh
- Fischer K (2018) Intracytoplamic sperm injection (ICSI) , digital photograph. Children born via IVF may face higher health risks as they get older. Link: http://bit.ly/319VJhj
- Alahmar T Ahmed (2019) Role of oxidative stress in male infertility: An updated review. Journal of Human Reproductive Sciences. Link: http://bit.ly/2ZAwSmN
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
