International Journal of Sexual and Reproductive Health Care
Hormonal Imbalance and Infectious Diseases in Female Infertility: A Cross-Sectional Study in Garoua, Cameroon
1Department of Biomedical Sciences, Faculty of Sciences, University of Ngaoundere, P.O. Box 455, Ngaoundere, Cameroon
2Ngaoundere Sunshine Diagnostics Research Laboratory, Ngaoundere, Cameroon
3Department of Obstetrics and Gynaecology, Faculty of Health Sciences, University of Buea, Cameroon
4Military Hospital of Garoua, Cameroon
5Department Food Engineering and Quality Control, University of Ngaoundere, P.O. Bo× 455, Ngaoundere,Cameroon
6Faculty of Medicine and Biomedical Sciences, University of Garoua, Cameroon
7Department of Biochemistry, Faculty of Science, University of Dcshang, Cameroon
8Department of Biological Sciences, Faculty of Sciences, University of Ngaoundere, P.O. Box 455, Ngaoundere, Cameroon
Author and article information
Cite this as
Yemele DM, Moune EZ, Eyong IM, Nodem SFS, Amina YDDA, Newe RD, et al. Hormonal Imbalance and Infectious Diseases in Female Infertility: A Cross-Sectional Study in Garoua, Cameroon. Int J Sex Reprod Health Care. 2025; 8(1): 001-008. Available from: 10.17352/ijsrhc.000050
Copyright License
© 2025 Yemele DM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Objective: Infertility is one of the most common reasons for consultation in gynecology services. This study aimed to assess the prevalence and associated factors of women’s infertility in Garoua City, North region, Cameroon in other to guide its prevention and treatment.
Materials and methods: This cross-sectional study was conducted in the gynecology services of Military and Regional Hospitals of Garoua and women coming for medical consultations were included. The variables studied including socio-demographic characteristics, style of life, and infertility statuses were assessed using a well-structured and validated questionnaire. Mycoplasma infections were assessed in vaginal swabs using Mycoplasma Culturing, Identification Kit whereas, chlamydia infections were detected in serum by enzyme-linked immunosorbent assay (ELISA) Kit. Hormone levels including follicle-stimulating (FSH), luteinizing (LH), and estradiol bloods serum were determined by Immunofluorescence.
Results: A prevalence rate of 9.52% (n = 62) of women infertility was recorded in this study. Primary infertility was more prevalent (58%) compared to secondary infertility (42%). The lifestyle mode of the women with infertility revealed the presence of those with high body mass index (22.6%), alcohol consumption (22.6%), and obesity (8.1%). U. urealyticum and C. trachomatis infections were the most common with 29.9% (n = 18) and 27.4% (n = 17) respectively. The hormonal mean values of 6.84 ± 5.41 IU/L, 12.44 ± 10.99 IU/L, 12.59 ± 6.21 pg/L, and 1.8 for FSH, LH, Estradiol and LH/FSH ratio respectively were found. Most women with infertility had normal hormone values of 79%, 74.2%, and 67.7% for FSH, Estradiol, and LH respectively (Table 5). High values of LH (27.4%) FSH (6.5%), and Estradiol (6.5%) and low values of Estradiol (19.4%), FSH (14.5%), and LH (4.8%) were also recorded.
Conclusion: Considering various associated factors for women’s infertility, this showed the important contribution of lifestyle mode, sexually transmitted diseases, the variability on the hormone balance, and LH/FSH ratio.
Infertility is one of the most important complications in gynecology and has been recognized as a global public health issue worldwide [1-3]. It is characterized as the incapacity to achieve pregnancy after one year of unprotected intercourse [1] and is divided into primary and secondary categories based on the presence or absence of a previous pregnancy [1]. Both female and male factors expose couples to infertility whereas, menstrual, ovulation disorders, and uterine factors are among the most common causes of female infertility [2]. Infertility has increased significantly by 0.37% per year [1] and prevalence ranges between 5% - 30% worldwide [1]. In 2022, the World Health Organization (WHO) reported that 15% of reproductive-aged couples worldwide are affected by infertility [4].
The causes of increasing infertility may include obstetrical history, smoking, and drinking patterns, changing family circumstances, having a child at a later age, the excessive use of contraception, illegal and legal abortion, adverse social conditions, climate-related factors, geographical areas, and possibly genetic variation [1]. In addition, some major risk factors have attracted much concern such as age, menstruation, BMI index, lifestyle, microbial infections, sex hormones, and environmental factors are considered to be leading to infertility [3,5].
Therefore, infertility prevalence depends on the age group examined, the definition of infertility, the geographical areas involved, the composition of the population studied, the selection criteria, the unit of measurement and relationship status, and the method of prevalence calculation used [1-6].
In addition, accurate assessment of the prevalence and various etiologies and or risk factors of infertility are required to plan appropriate strategies for prevention, treatment, and management of its health and socio-economic consequences.
In Cameroon, the prevalence rate of infertility ranges from 15% to 30% and secondary infertility is twice as common as primary infertility [5]. A study by Egbe, et al. [7] conducted in Douala, reported an infertility prevalence of 19.2% and mentioned that sexually transmitted diseases, dysmenorrhea, and abortion history increased the risk of infertility. However, there is a lack of data on infertility in the North Region. This study aimed to investigate the prevalence of infertility and analyzed the socio-demographic, behavioral, and reproductive factors associated with infertility in Cameroon to guide its prevention and treatment.
Materials and methods
Study population and data collection
A cross-sectional and descriptive study was conducted involving two public health clinics in Garoua, Cameroon from July to October 2022 to investigate the prevalence and risk factors associated with infertility in Garoua, North region, Cameroon. The study population consisted of probable infertile couples referring to the gynecology services of the Military and Regional Hospitals of Garoua. These hospitals were selected since they are the main and only hospitals that provide infertility services in the North Region. Non-probabilistic and consecutive sampling method was used. The inclusion criteria included all probable infertile women referred to both hospitals for specialized medical consultation, and the exclusion criteria included the unwillingness to participate in the study. The data were collected by a researcher-made questionnaire based on previous studies. The questionnaires were completed using interviewing in a closed room after ensuring the confidentiality of the information and the way the information would be used in this research. A part of the questionnaire, which included specialized questions, was extracted from the medical documents of the subjects and entered into the questionnaire. The questions were in two parts; the first part included demographic pieces of information about women including age, education level, occupation, body mass index (BMI), presence of underlying diseases, smoking, alcohol consumption, etc. The fertility data of the couples were also recorded in the second part. The infertility data included information on the type (primary, secondary) and duration of infertility, and regularity in menstruation. After this step, women presenting infertility were selected and subjected to microbial and hormone parameters assessment.
Hormone titration
Four milliliters of venous blood were collected into a dry tube for sex hormone determination including Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), and estradiol using an Immunofluorescent assay technique and reagents (Finecare diagnostic products, Dhaka, Bangladesh). The test uses a sandwich immunodetection method where the detector antibodies in the buffer bind to antigens in the sample forming antigen-antibody complexes, and migrate onto nitrocellulose matrix to be captured by the other immobilized antibodies on a test strip. The complexes will lead to a fluorescence signal by detector antibodies which is processed by the instrument to show hormone concentration in a sample.
Concerning quality control, each Finecare TM LH Rapid quantitative test cartridge contains internal control that satisfies routine quality control requirements. This internal control was purchased each time a test sample was done. The control test cartridge was inserted and read by the Finecare TM FIA System. An invalid result from the internal control causes an error message on the Finecare TM FIA System indicating that the test should be repeated.
Microbial analysis
Genital mycoplasmas detection: High vaginal swabs were taken from each subject enrolled in the study using sterile cotton swabs. Each swab was inoculated to one diluent vial (provided by the kit). Diluent vials were then recapped and sent to the laboratory for processing. Genital Mycoplasmas detection was done using Mycoplasma Culturing, Identification, Enumeration, and Susceptibility Kit (Mycoplasma IES kit – Autobio Diagnostics – China). The Mycoplasma IES kit is based on cultivation and biochemical reactions. Urea is decomposed by the urease enzyme produced by U. urealyticum with the release of NH3. For the detection of M. hominis, arginine is decomposed by the Arginase enzyme produced by the organism and releases NH3. Then NH3 causes an increased pH of the liquid medium. The corresponding color change of the indicator was used to judge the result.
Briefly, culture media were prepared by mixing the freeze-dried powder vial (peptone of bovine origin and beef heart infusion) and the inoculated diluent vials (both provided by the kit). The procedure was done according to the manufacturer’s instructions. Each inoculated diluent vial was added to one of the freeze-dried powder vials (both were provided by the kit). After well shaking and complete dissolution, 100 µL of the mixture was added to each well of the strip by automatic pipetting. Strip was then shaken gently then each well was covered with one drop of mineral oil. The strip was then covered and incubated at 37 °C for 24 h. The change in color of the well to red color indicated a positive reaction and microbial growth.
Chlamydia trachomatis genital infection: Detection of Chlamydia trachomatis Specific IgG by ELISA Circulating anti- C. trachomatis IgG antibodies was detected in serum of women by ELISA technique (enzyme-linked immunosorbent assay) using DRG Chlamydia Trachomatis IgG Kit (DRG International Inc., U.S.A.). The kit provides all necessary materials for the qualitative determination of IgG-class antibodies to C. trachomatis in serum. The DRG C. trachomatis IgG ELISA kit is a solid phase ELISA.
The manufacturer’s instructions were followed for sample collection and preparation, assay technique, calculation, and result interpretation. Venipuncture was used to collect blood, which was then allowed to clot before the serum was separated at room temperature using centrifugation. The specimens were kept at –20°C. Every patient sample was diluted 1:100 with sample diluent before assaying. To utilize 10 µL of specimen, for example, mix 1 mL of sample diluent thoroughly, let it stand for 15 minutes, and then mix it again before using. The wash solution was diluted (1/20) with new, germ-free redistilled water before the test was started. Before being used, all of the chemicals and the necessary quantity of strips were allowed to come to room temperature. The kit’s dissemination and identification strategy was thoughtfully created for each specimen and control.
The following formula was used for absorbance (A) values: ,
Where, OD: Optical Density; CO: Cut-off value
- Interpretation of the results waPositive, when Patient absorbance values more than 10% above CO,
- Grey zone: when Patient absorbance values from ± 10% (CO), the test was repeated 2 - 4 weeks later - with new patient samples. Results in the second test again in the grey zone conclude to negative,
- Negative: Patient absorbance values more than 10% below CO.
Ethical consideration
Research authorization was obtained from the University of Ngaoundere, the Biomedical Sciences Department, and the ethical committee respectively. Administrative authorization was obtained from Garoua Regional Health Office and Garoua Regional Hospital. Written informed consent was obtained from each of the woman studies. The research method, objectives, and the period of study were explained to the subjects. They were then enrolled in the study and were asked to reply to questions honestly. Informed consent of each adult participant was obtained before blood sample collection.
Statistical analysis
The data were analyzed using XLStat Addinsoft software version 16.0. The mean and standard deviation (SD) were used to determine and describe the quantitative variables. Distributions of quantitative variables were analyzed with the Shapiro-Wilk test. Mean values of continuous variables were compared with non-parametric tests: Mann-Whitney U-test. Distributions of qualitative variables were compared with the chi-square test and Fischer exact test. The threshold of statistical significance for all the tests was set at p < 0.05.
Results and discussion
Socio-demographic characteristics of respondents and prevalence of infertility
Out of 651 consultations made at the gynaeco-obstetrics department of the Garoua Regional Hospital and the Garoua Military Hospital from July to October 2022, 62 (9.52%) women were consulted for infertility and were included in this study and the socio-demographic characteristics are illustrated in Table 1. The table shows that the most represented age group was 20-30 years (69.35%) with the mean age of the infertile of 25 years. Married women were the most represented (98.39%). The majority (70.97%) were housewives and 82.26% were monogamous. Similarly, in terms of type of infertility, primary infertility (58%) predominated over secondary infertility (42%). Concerning the age group our results are slightly of those Ahmadi, et al. [8] in Iran reporting the predominance of mean age of the infertile of 31 (19–40) years. Other studies reported the dominance of the 30 to 39 years age group [9,10].
Infertility is a main socio-economic, demographic, reproductive health, and clinical issue affecting millions of people of reproductive age worldwide. Infertility also can cause significant financial loss and emotional stress [11]. Despite this reality, infertility in low-resource countries is still a poorly researched and ignored subject [2]. This cross-sectional study demonstrated that the infertility prevalence in a sample of women in Garoua City was about 9.62%. These results are less than the reports from Sudan, 13% [12], Gambia, 14.3% [13] Cameroon, 19.2% [7], 15% - 30%, [5] Korea, 19.42% [14], Ethiopia, 20% [15], Nigeria, 23.9% [16], Buea, Cameroon, 24% [10], Ukraine, 25.4% [6].
In this study, a predominance of primary infertility (58%) which is the same as in other studies in Sudan [12]. In contrast, our results are different from those of Tiagha, et al. [10] in Buea, Cameroon (31% versus 69%) several authors and particularly in most Middle East and North Africa [13,16,17] reporting a predominance of secondary infertility. This difference in the percentage of women with primary or secondary infertility in these countries is likely influenced by the causes of infertility in that region. However, we also noted the high proportion of primary infertility which is mostly associated with high bacterial infections such as mycoplasma, chlamydia, gonorrhea, and syphilis [9,10].
Our study revealed age extremes of 15 and 40 years with the mean age of infertility ranging from 26 to 31 years. This result is similar to those found by authors [10,13,16]. This may be explained by the fact that women are concerned about their fertility because at this age the probability of being fertile or conceiving is high. Married women were the most represented (98.39%) and this could be explained by the fact that a given bird in a household is more solicited. Of the 62 cases, 27 women had attended secondary school. The majority (82.26%) of the patients were monogamous. This can be explained by the fact that young couples with medium levels of education have few social and financial constraints, and are much more concerned about their health. On the other hand, in polygamous households, the increase in family size increases the couple’s financial pressure. About 70.97% of the patients were housewives, which is close to the results of Dattijo, et al. who found 62.7% [16]. This can be explained by the socio-cultural habits of the population in this part of the country, where women tend to stay at home to look after the family and children.
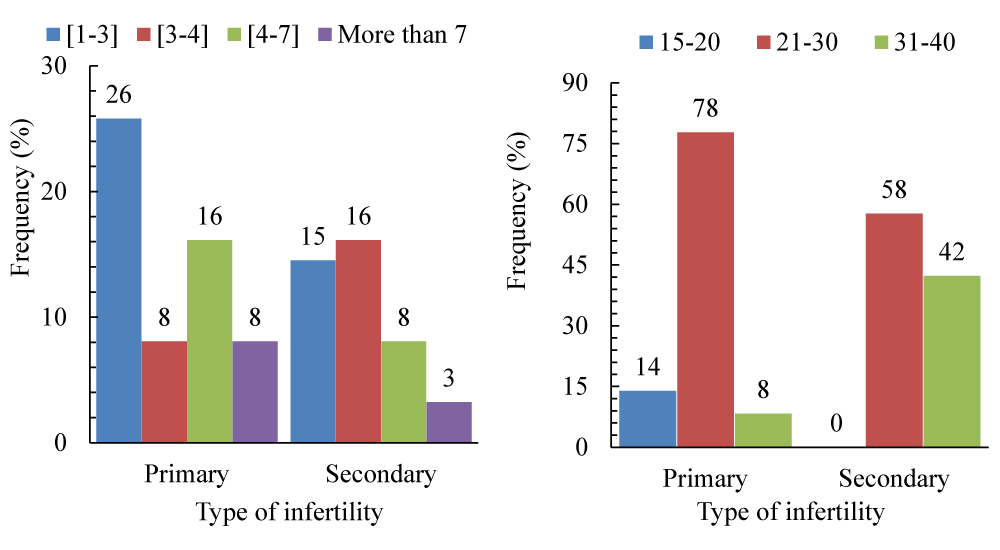
The distribution of the type of infertility with the duration of infertility (Figure 1a) and age class (Figure 1b). However, we observe that patients with primary infertility consult doctors after 1 to 2 years of living together (5.81%) compared to 14.52% in secondary infertility; on the other hand, patients with secondary infertility consult after 3 to 4 years of living together, i.e., 16.13% compared to 8.06% in primary infertility (p > 0.05). On the other hand, we note that secondary infertility is mostly found in women aged from 31 to 40 years.
In the present study, there was a significant association between level of education and duration of infertility. Women with a medium or low level of education had a longer duration of infertility, whereas women with a high level of education went to the hospital very quickly for treatment. The observations have been reported by authors [18].
Associated factors of infertility couples
Lifestyle: The lifestyle mode of the women with infertility revealed the presence of about 60% of non-recommended habits (Table 2) characterized by a high body mass index (22.6%), alcohol consumption (22.6%), and obesity (8.1%).
When we look at the relationship between patients’ lifestyle and socio-demographic characteristics, we find that only patients’ age and education level had a significant association with alcohol consumption (p = 0.0016), x2 = 12.86) and weight loss (p = 0.013; x2 = 10.78) respectively (Table 3). Authors reported the place of some medical history and examination including overweight, obesity, and alcohol consumption as causes of infertility [9,19]. In this study, overweight and alcohol-consuming women were the most represented with the same frequency of 22.6%. This is in agreement with literature where alcohol consumption induced reduction of the FSH secretion, and suppression of folliculogenesis and ovulation [19].
Obesity and high BMI, are recognized as causes of menstrual and ovulation disorders and it is suggested that hormonal imbalance resulting in certain body features can be linked to the pathogenesis of polycystic ovary syndrome, PCOS [20]. The same authors found a significant association of PCOS among overweight and obese patients compared to those with normal BMI.
Microbial factors: Concerning microbial infections, U. urealyticum and C. trachomatis infections were the most common with 29.9% (n = 18) and 27.4% (n = 17) respectively, while 29.0% (n = 18) of the latter were not infected (Table 4).
In the present study prevalence of anti-C. trachomatis IgG in asymptomatic infertile was 27.4%. Specific anti- C. trachomatis IgG, infertile women, determined by immunoenzymatic assay, was 31.1% in Egypt [21] and 39% in Ghana [22] which was similar to the present study. In contrast, these results were higher than those of the United States of America estimated to range from 5% to 15%, and those of the United Kingdom at 16% [23]. These were also higher than those of Piscopo, et al. in Brazil, 3.7%, [24], Al-Ramahi, et al. in Jordan, 3.9% [25], Li, et al. in China 5.9% [26], Alfarraj, et al. and Kamel. 8.0% and 9.84% respectively in Saudi Arabia [23,27], Nwankwo and Sadiq in Kano, Nigeria, 9.6% [28], Sameni, et al. in Tehran, 13.8%, [29], Ahmadi, et al. in Iran, 14%, [8] Oloyede et al. in Lagos, Nigeria, 18.2% [30].
Among Cameroonian infertile women, the prevalence of C. trachomatis infection ranged from 7.0% [5], 50.3% [7] to 92% [31] depending on the method of diagnosis, the study area, the sampling size, and procedures. Authors of all previous studies concluded that C. trachomatis plays a role in infertility but did not significantly differ by case/control status. Several authors reported that bacterial infections such as C. trachomatis, M. genitalium, Neisseria gonorrhoeae, and U.urealyticum could be associated with the infertility of couples [5], [10,20,29]. Chlamydia trachomatis is the most common bacterial cause of sexually transmitted infections and authors revealed that genetic predisposition and host immune response play important roles in the pathogenesis of long-term complications [21-31].
Concerning mycoplasma infections the prevalence rate of 29.9% (U. urealyticum) and 4.8% (M. hominis) were recorded in this study. Concerning U. urealyticum, the prevalence of 3.23% in Nigeria [32], 4.28% in Iran [33], 9.0% in Brazil [24], 9% in Douala, Cameroon [7], and 23.1% in Tehran [29] lower than those of this study was recorded. Simultaneously, the M. hominis infection prevalence of 3.14% in Iran [33], 5.7% in Brazil [24], 6.45% in Nigeria [32] and 12.3% in Douala, Cameroon [7] were registered. Excepted both species studied in the present works, Ureaplasma parvum and Mycoplasma genitallium have always been identified among infertile women. The prevalence rate of M. genitalium of 8% in Iran [8], and 13.8% in Tehran [29] was recorded.
In the present study, we noticed the predominance of mycoplasma infections compared to chlamydia among infertile women. The same observation has been reported by other authors [5,24,29] while, others reported the adverse results [7,8]. Hence, mycoplasma and chlamydia infections would be one of the main causes of infertility among patients received for infertility in the Regional and Military Hospitals of Garoua. This is explained by the fact that at the beginning, the infection was asymptomatic and the women let the disease linger without knowing it, and this affected their fertility.
The statistical analysis between these infectious agents and socio-demographic characteristics was performed and no significant association was obtained (Table 3). However, these infections are much more prevalent in patients aged 21–30 years, with secondary education, and married in monogamous relationships. Similarly, none of the infections studied had a significant impact on infertility duration (p = 0.622), cycle regularity (p = 0.642), and miscarriage (p = 0.657) (Table 5).
Hormonal factors: The hormonal parameters were explored in this study and the mean values were 6.84 ± 5.41 IU/L, 12.44 ± 10.99 IU/L, 12.59 ± 6.21 pg/L, and 1.8 for FSH, LH, Estradiol, and LH/FSH ratio of respectively were found. Most women with infertility had normal hormone values of 79%, 74.2%, and 67.7% for FSH, Estradiol, and LH respectively (Table 6). Nevertheless, high values of LH (27.4%) FSH (6.5%), and Estradiol (6.5%) and low values of Estradiol (19.4%), FSH (14.5%), and LH (4.8%) were also recorded.
The mean values of 6.84, 12.44 IU/L, and 12.59 Pg/L for FSH, LH and Estradiol respectively and the LH/FSH ratio of 1.8 was obtained in women infertility. The similar values of FSH (6.40 and 6.46 IU/L), LH (8.35 and 10.15 IU/L), LH/FSH ratio (1.2 and 1.7), and the higher estradiol (28.10 pg/L) were obtained by authors [20], [34]. These results revealed an increased level of LH and estradiol and decreased FSH levels in women with infertility. Similar observations have been carried out in the literature using other hormones and explained that hormonal imbalance in women with infertility characterized by increased levels of AMH and LH, estradiol, and testosterone and decreased FSH levels could be mainly caused by polycystic ovary syndrome [20] that has not been expected in this study.
Female infertility occurs in about 37% of all infertile couples and authors mentioned that ovulatory disorders account for more than half of these [35]. Polycystic ovary syndrome is a highly prevalent endocrine and metabolic disorder among reproductive-aged women, leading to female infertility and characterized by oligomenorrhea or amenorrhea, hyperandrogenism, [20] and variability in LH: FSH ratio [34]. Other factors could explain the abnormal (low or high) values obtained. For example, Petrglia, et al. [19] reported that alcohol consumption induced reduction of the FSH secretion, and suppression of folliculogenesis and ovulation. Similarly, Khmil, et al. [20] observed that the LH/FSH ratio was 30.35% higher in women with Class 2 obesity than in the group of women with normal weight. De Pergola, et al. [36] reported that fertile women with high body mass index have lower FSH, LH, and estradiol levels in the early follicular phase, with a possible direct inhibitory effect on gonadotropin and estradiol production, and other hormones leading to infertility. Statistical analysis with some socio-demographic parameters revealed no significant relationship with age, duration of infertility, and type of infertility (p > 0.05). However, it was found that high values of all hormones studied were found in patients aged 21-30 years and with primary infertility (Table 7). Regarding the relationship between the hormonal profile and the regularity of the menstrual cycle, there was no significant association. Regarding LH and type of infertility, we found no significant relationship. This result is similar to other studies reporting the absence of a relationship between LH level and type of infertility [37].
Conclusion
The present study aimed at detecting some risk factors for infertility among women undergoing medical consultations with gynecologists in the city of Garoua. We observed that about 9.52% of these women suffered from infertility and the main risk factors included excessive alcohol intake, Ureaplasma urealyticum, Mycoplasma hominis and Chlamydia trachomatis infections associated with slight variations in hormone levels (LH and Oestradiol). These results highlight the need to increase awareness campaigns on infection prevention methods.
Data availability
The data used to support the findings of this study are available from the corresponding authors on request.
We extend our sincere thanks to the women who agreed to be part of this study, the University of Ngaoundere, and the Ngaoundere Sunshine Diagnostic Laboratory for providing us with all the facilities to carry out this work.
- Mirzaei M, Namiranian N, Firouzabadi RD, Gholami S. The prevalence of infertility in 20-49 years women in Yazd, 2014-2015: A cross-sectional study. Int J Reprod BioMed. 2018;16(11):683–688. Available from: https://pubmed.ncbi.nlm.nih.gov/30775683/
- Obeagu EI, Njar VE, Obeagu GU. Infertility: Prevalence and consequences. Int J Curr Res Chem Pharm Sci. 2023;10(7):43–50. Available from: https://ijcrcps.com/pdfcopy/2023/july2023/ijcrcps5.pdf
- Fupare S, Gadhiya BM, Jambhulkar RK, Tale A. Correlation of thyroid hormones with FSH, LH, and prolactin in infertility in the reproductive age group women. Int J Clin Biochem Res. 2015;2(4):216–222. Available from: https://doi.org/10.22159/ajpcr.2023.v16i5.47027
- WHO. Infertility. Available from: https://www.who.int/health-topics/infertility#tab=tab_1.
- Mbah CE, Jasani A, Aaron KJ, Akoachere JF, Tita ATN, Geisler WM, et al. Association between Chlamydia trachomatis, Neisseria gonorrhea, Mycoplasma genitalium, and Trichomonas vaginalis and secondary infertility in Cameroon: A case-control study. PLoS One. 2022;17(2):e0263186. Available from: https://doi.org/10.1371/journal.pone.0263186
- Salmanov AG. Prevalence and risk factors of infertility in Ukraine: results of a multicenter study (2019-2021). Wiad Lek. 2022;75(5):1058–65.
- gbe TO, Mbaki CN, Tendongfor N, Temfack E, Belley-Priso E. Infertility and associated factors in three hospitals in Douala, Cameroon: A cross-sectional study. Afr Health Sci. 2020;20(4):1985–1995. Available from: https://doi.org/10.4314/ahs.v20i4.57
- Ahmadi K, Moosavian M, Jalal M, Pouresmaeil O, Afzali M. Prevalence of Chlamydia trachomatis, Ureaplasma parvum, and Mycoplasma genitalium in infertile couples and the effect on semen parameters. Ethiop J Health Sci. 2023;33(1):133–142. Available from: https://doi.org/10.4314/ejhs.v33i1.17
- Madziyire MG, Magwali TL, Chikwasha V, Mhlanga T. The causes of infertility in women presenting to gynecology clinics in Harare, Zimbabwe: A cross-sectional study. Fertil Res Pract. 2021;7(1):1–8. Available from: https://doi.org/10.1186/s40738-020-00093-0
- Tiagha AR, Ngemenya M, Enoh JE, Nguedia JCA. A retrospective study of the prevalence of female infertility in the Southwest Region, Cameroon. Open J Obstet Gynecol. 2020;10(12):1728–1740. Available from: https://www.scirp.org/journal/paperinformation?paperid=106122
- Salmanov AG, Vitiuk AD, Kovalyshyn OA, Baksheev SM, Kutytska TV, Korniyenko SM, et al. PREVALENCE AND RISK FACTORS OF INFERTILITY IN UKRAINE: RESULTS A MULTICENTER STUDY (2019-2021). Wiad Lek. 2022;75(5 pt 2):1234-1241. Available from: https://doi.org/10.36740/wlek202205202
- Abdullah AA, Ahmed M, Oladokun A. Prevalence of infertility in Sudan: A systematic review and meta-analysis. Qatar Med J. 2021;2021(3):1–13. Available from: https://doi.org/10.5339/qmj.2021.47
- Anyanwu M, Idoko P. Prevalence of infertility at the Gambian Teaching Hospital. Women’s Heal Gynecol. 2017;3(2):7–10. Available from: https://scientonline.org/open-access/prevalence-of-infertility-at-the-gambian-teaching-hospital.pdf
- Lee HJ, Han JY, Choi HZ, Na BJ. Infertility prevalence and associated factors among women in Seoul, South Korea: A cross-sectional study. Clin Exp Obs Gynecol. 2023;50(3):1–9. Available from: https://doi.org/10.31083/j.ceog5003054
- Legese N, Tura AK, Roba KT, Demeke H. The prevalence of infertility and factors associated with infertility in Ethiopia: Analysis of Ethiopian Demographic and Health Survey. PLoS One. 2023;18(10):1–16. Available from: https://doi.org/10.1371/journal.pone.0291912
- Dattijo LM, Andreadis N, Aminu B, Umar N, Black K. The prevalence and clinical pattern of infertility in Bauchi, Northern Nigeria. Trop J Obs Gynaecol. 2016;33(1). Available from: https://www.ajol.info/index.php/tjog/article/view/135965
- Abdallah E, Osama TA. Infertility in the Middle East and North Africa region: A systematic review with meta-analysis of prevalence surveys. Libyan J Med Sci. 2018;2(2):37–44. Available from: https://journals.lww.com/ljms/fulltext/2018/02020/Infertility_in_the_Middle_East_and_North_Africa.2.aspx
- Roupa Z, Polikandrioti M, Sotiropoulou P, Faros E, Koulouri A, Wozniak G, Gourni M. Causes of infertility in women at reproductive age. Health Sci J. 2009;3(2):80–87. Available from: https://www.itmedicalteam.pl/articles/causes-of-infertility-in-women-at-reproductive-age.pdf
- Petraglia F, Serour GI, Chapron C. The changing prevalence of infertility. Int J Gynecol Obstet. 2013;123:4–8. Available from: https://doi.org/10.1016/j.ijgo.2013.09.005
- Khmil M, Khmil S, Marushchak M. Hormone imbalance in women with infertility caused by polycystic ovary syndrome: Is there a connection with body mass index? Open Access Maced J Med Sci. 2020;8:731–737. Available from: https://doi.org/10.3889/oamjms.2020.4569
- Siam EM, Hefzy EM. The relationship between antisperm antibodies prevalence and genital Chlamydia trachomatis infection in women with unexplained infertility. Middle East Fertil Soc J. 2012;17(2):93–100. https://pubmed.ncbi.nlm.nih.gov/22574496/
- Siemer J, Theile O, Larbi Y, Fasching PA, Danso KA, et al. Chlamydia trachomatis infection as a risk factor for infertility among women in Ghana, West Africa. Am J Trop Med Hyg. 2008;78(2):323–327. Available from: http://dx.doi.org/10.4269/ajtmh.2008.78.323
- Alfarraj DA, Somily AM, Alssum RM, Abotalib ZM, El-Sayed AA, Al-Mandeel HH. The prevalence of Chlamydia trachomatis infection among Saudi women attending the infertility clinic in central Saudi Arabia. Saudi Med J. 2015;36(1):61–66. Available from: https://doi.org/10.15537/smj.2015.1.9967
- Piscopo RC, Guimarães RV, Ueno J, Ikeda F, Bella ZIJ, Girão MJ, et al. Increased prevalence of endocervical Mycoplasma and Ureaplasma colonization in infertile women with tubal factor. JBRA Assist Reprod. 2020;24(2):152–157. Available from: https://doi.org/10.5935/1518-0557.20190078
- Al-Ramahi M, Mahafzah A, Saleh S, Fram K. Prevalence of Chlamydia trachomatis infection in infertile women at a university hospital in Jordan. East Mediterr Health J. 2008;14(5):1148–1154. Available from: https://pubmed.ncbi.nlm.nih.gov/19161088/
- Li C, Tang W, Ho HC, Ong JJ, Zheng X, Sun X, et al. Prevalence of Chlamydia trachomatis among pregnant women, gynecology clinic attendees, and subfertile women in Guangdong, China: A cross-sectional survey. Open Forum Infect Dis. 2021;8(6). Available from: https://doi.org/10.1093/ofid/ofab206
- Kamel RM. Screening for Chlamydia trachomatis infection among infertile women in Saudi Arabia. Int J Womens Health. 2013;5(1):277–284. Available from: https://www.tandfonline.com/doi/full/10.2147/IJWH.S46678
- Nwankwo EO, Sadiq MN. Prevalence of Chlamydia trachomatis infection among patients attending infertility and sexually transmitted diseases clinic (STD) in Kano, north western Nigeria. Afr Health Sci. 2014;14(1):672–678. Available from: https://www.ajol.info/index.php/ahs/article/view/107252
- Sameni F, Zadehmodarres S, Dabiri H, Khaledi M, Nezamzadeh M. Evaluation of Ureaplasma urealyticum, Chlamydia trachomatis, Mycoplasma genitalium, and Neisseria gonorrhoeae in infertile women compared to pregnant women. J Obstet Gynaecol (Lahore). 2022;42(6):2151–2155. Available from: https://doi.org/10.1080/01443615.2022.2035328
- Oloyede OAO, Fakoya TA, Oloyede AA, Alayo AM. Prevalence and awareness about chlamydial infection in women undergoing infertility evaluation in Lagos, Nigeria. Int J Heal Res. 2009;2(2):157–162. Available from: https://www.ajol.info/index.php/ijhr/article/view/55412
- D'Amico M, Mbah JCE, Gupta K, Dionne JA, Akoachere JF, Nguedia JCA, Van Der Pol B, Geisler WM. Chlamydia trachomatis–Specific Antibody Responses in Women in Cameroon With Secondary Infertility. Sex Transm Dis. 2023;50(11):30–33. Available from: https://journals.lww.com/stdjournal/abstract/2023/11000/chlamydia_trachomatis_specific_antibody_responses.14.aspx
- Ezeanya-Bakpa CC, Agbakoba NR, Oguejiofor CB, Enweani-Nwokelo IB. Sequence analysis reveals asymptomatic infection with Mycoplasma hominis and Ureaplasma urealyticum possibly leading to infertility in females: A cross-sectional study. Int J Reprod Biomed. 2021;19(11):951–958. doi: https://doi.org/10.18502/ijrm.v19i11.9910
- Seifoleslami M, Safari A, Khameneie MK. Prevalence of Ureaplasma urealyticum and Mycoplasma hominis in High Vaginal Swab Samples of Infertile Females. Iran Red Crescent Med J. 2015;17(12):0-4. Available from: https://doi.org/10.5812/ircmj.16823
- Malini NA, George KR. Evaluation of different ranges of LH: FSH ratios in polycystic ovarian syndrome (PCOS) – Clinical based case control study. Gen Comp Endocrinol. 2018;260:51–57. Available from: https://doi.org/10.1016/j.ygcen.2017.12.007
- Unuane D, Tournaye H, Velkeniers B, Poppe K. Endocrine disorders & female infertility. Best Pract Res Clin Endocrinol Metab. 2011;25(6):861–873. Available from: http://dx.doi.org/10.1016/j.beem.2011.08.001
- De Pergola G, Maldera S, Tartagni M, Pannacciulli N, Loverro G, Giorgino R. Inhibitory effect of obesity on gonadotropin, estradiol, and inhibin B levels in fertile women. Obesity. 2006;14(11):1954–1960. Available from: https://doi.org/10.1038/oby.2006.228
- Ben-Chioma and Tamuno-Emine. Evaluation of Female Fertility Hormone Profile in Women with Primary and Secondary Infertility. Int J Sci Res. 2013;4(October):2319–7064. Available from: https://www.ijsr.net/archive/v4i10/SUB159134.pdf
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
