Open Journal of Pediatrics and Child Health
How are pediatricians treating molluscum contagiosum? results from in-depth interviews
Martina Cartwright1*, Tomoko Maeda-Chubachi1*, Carolyn Enloe1, Stephen Stripling2 and Adelaide Hebert3
2Coastal Pediatric Associates, Charleston, SC, USA
3UTHealth McGovern Medical School, Houston, TX, USA
Martina Cartwright, Pelthos Therapeutics., Durham, NC, USA, E-mail: [email protected]
Cite this as
Cartwright M, Maeda-Chubachi T, Enloe C, Stripling S, Hebert A (2024) How are pediatricians treating molluscum contagiosum? results from in-depth interviews. Open J Pediatr Child Health 9(1): 040-044. DOI: 10.17352/ojpch.000056Copyright
© 2024 Cartwright M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Molluscum contagiosum is a common, often persistent viral skin infection affecting children, and spread by infected individuals or objects. No molluscum management guidelines exist and only two FDA treatments have been approved in the last year, thus placing pediatricians in a clinical conundrum if molluscum treatment is warranted. When MC is particularly bothersome or persistent, parental anxiety may influence treatment. In-depth interviews with 25 pediatricians provided insight into the current MC treatment landscape and drivers of therapeutic intervention. This study reveals the important influence parental anxiety, patient characteristics, and parental involvement have on pediatrician’s molluscum management and treatment intervention practices. Professional society endorsed molluscum management guidelines and the use of safe and efficacious FDA-approved therapies would fill therapeutic gaps.
Abbreviations
FDA: Food and Drug Administration; MC: Molluscum Contagiosum; SD: Standard Deviation
Introduction
Molluscum contagiosum is a viral skin infection caused by the Molluscipoxvirus and is characterized by flesh-colored bumps that are round, dome-shaped, and usually umbilicated [1]. Lesions commonly appear on the face, trunk, and extremities of children. The virus resides in the human epidermis and is transmitted by skin-to-skin contact with infected persons or contaminated objects; scratching may cause lesions to become more widespread or appear in other areas of the body through auto-inoculation.
Pediatricians may diagnose molluscum in either an annual well-child visit or through an office visit spurred by parental or caregiver concern. Per the Red Book®, the standard of care for the treatment of MC infections is to “wait and see” or “watch and wait,” with an expectation that lesions will spontaneously resolve in a matter of months [2]; however, some episodes last months or years [3]. Treatment may be warranted for resistant MC cases, younger patients with large or numerous lesions, lesions in sensitive body regions, patients with bothersome symptoms such as itching, redness, secondary infection, and/or when there is concern about the potential for scarring or spreading to close contacts, such as siblings [4]. However, there is no consensus on the management of molluscum in children and adolescents, and until 2023, there were no United States Food and Drug Administration (FDA)–approved treatments. The lack of widely available treatment options places pediatricians in a clinical conundrum, particularly if a parent or caregiver strongly advocates intervention.
The current gold standard for the treatment of MC is the physical destruction of lesions using curettage or cryotherapy. Chemical agents designed to trigger local inflammation are used to treat MC including cantharidin, off-label tretinoin, 25%–50% trichloroacetic acid, silver nitrate, potassium hydroxide, and imiquimod [2].
The most common treatments, cryotherapy, curettage, and cantharidin application, require multiple in-office visits, which can be time-consuming depending on the number and location of lesions. Further, potential adverse outcomes with cantharidin, including pain, burning, and discomfort after treatment, are more likely with the improper application or inadequate post-application care (ie, washing the cantharidin off a few hours following treatment) [2].
In the absence of MC treatment guidelines, and the off-label use of unapproved therapies, it is unknown how pediatricians typically manage and select therapies to treat children with MC. It is hypothesized that two recently approved treatments will fill an unmet MC therapeutic gap [5-7].
The purpose of this structured, in-depth interview of 25 pediatricians was to understand MC diagnosis and management strategies, gather information on factors that impact treatment decision-making, and gain physicians’ perspectives on current treatment options and therapeutic gaps.
Materials and methods
From May 12 to 27, 2022, Syneos Health Consulting conducted in-depth telephone interviews with a cross-section of 25 pediatricians in the United States. This market research interview consisted of 35 questions (Table 1) to collect information about the location (state) of practice, practice characteristics (including the percentage of practice devoted to patient care), practice type, and monthly number of patients with MC. Pediatricians were also queried about their current MC diagnostic criteria, treatment practices (including a wait-and-see approach in which no treatment was initiated), or if the practitioner tended to treat patients with MC, use of pharmacologic therapy (eg, over-the-counter, prescription), physical removal, or both. Pediatricians were classified into one of three primary categories based on their MC management strategy: wait-and-see manager, treater, or referrer. Wait-and-see captured pediatricians who initiated no treatment upon MC diagnosis for most patients. Referrers captured pediatricians who referred > 50% of their patients with MC to a specialist (e.g., dermatologist) or another physician. Treaters captured pediatricians who treated > 50% of their patients with MC. Interview responses were aggregated by category, and descriptive statistics were reported.
Results
Of the 25 pediatricians interviewed, 13 (52%) were female and 12 (48%) male; 7 (28%) were from the Midwest, and 6 (24%) each from the Northeast, South, and West. Nine (36%) were in a single specialty group practice; 7 (28%) in a multi-specialty group; 6 (24%) in private practice, and 3 (12%) in an Integrated Delivery Network. An average of 97.5% of the practices were devoted to patient care with a mean (±SD) of 72 (±57.6) (median, 50) patients with MC per seen month.
Most pediatricians (≥ 90%) interviewed relied primarily on their residency training to manage patients with MC, followed by clinical experience and colleague consultations. Respondents estimated that nearly two-thirds (62%) of their patients with MC were diagnosed during a parent/caregiver-initiated office visit for lesion evaluation, and the remaining third (38%) were diagnosed during a well-child visit. The average time between a parent/caregiver first noticing the lesions and seeking an office visit was 2 to 4 months. More consultations occurred during warmer seasons. Pediatricians reported most parents were unfamiliar with molluscum unless through previous experience with an infected child’s sibling. All respondents (n = 25, 100%) reported that the MC diagnosis was visual, without laboratory testing or biopsy. Pediatricians reported the most common lesion location(s) were extremities, including legs, arms, forearms, and hands (60%, n = 15), followed by the trunk (56%, n = 14), face/neck (40%, n = 10), sensitive locations, including the groin, axillary region, and behind the knees (16%, n = 4), and variable (4%, n = 1). Among pediatricians interviewed, a typical patient with MC was between 2 and 8 years of age with between 10 to 24 lesions.
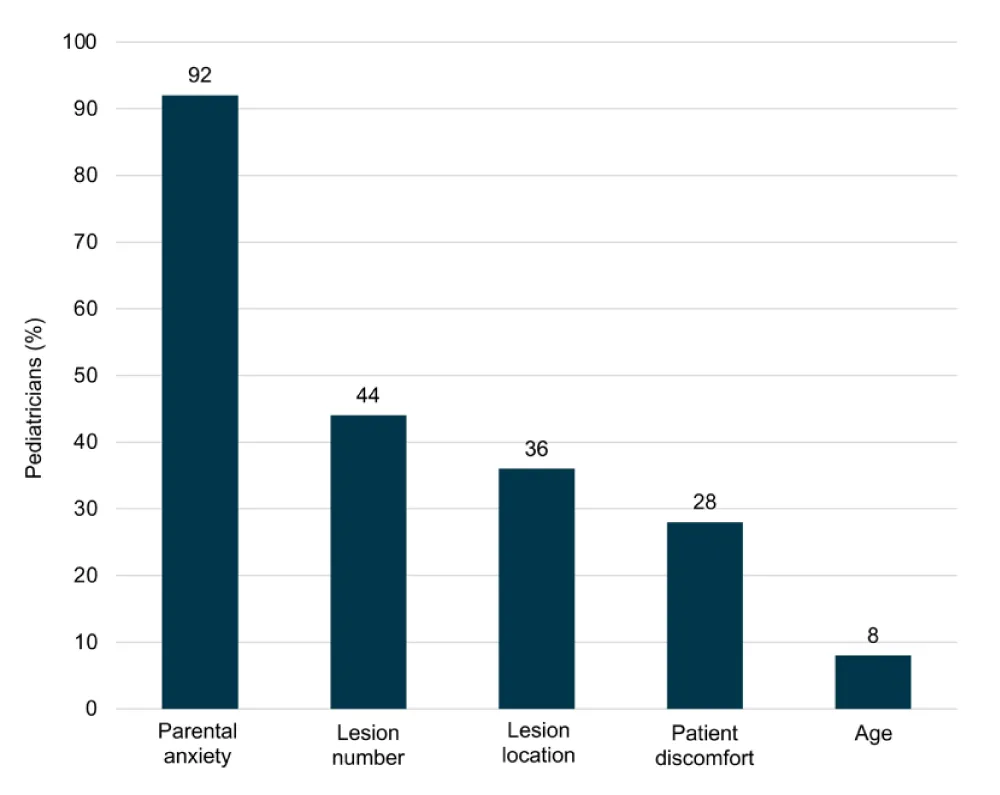
Most pediatricians interviewed were classified as wait-and-see managers (68%, 18/25) and 32% (7/25) as treaters. When asked “What goes into your decision to treat or not to treat?” parental anxiety was reported as a key treatment decision driver (n = 23/25), followed by lesion number (n = 11/25), lesion location, (n = 9/25), patient discomfort (n = 7/25), and age (n = 2/25) (Figure 1).
Referrals to a specialist, like a dermatologist, were limited and in response to parents’ desire for a referral or in cases where treatment may be challenging due to lesion location, high risk of patient discomfort, or MC treatment is not offered by the pediatrician.
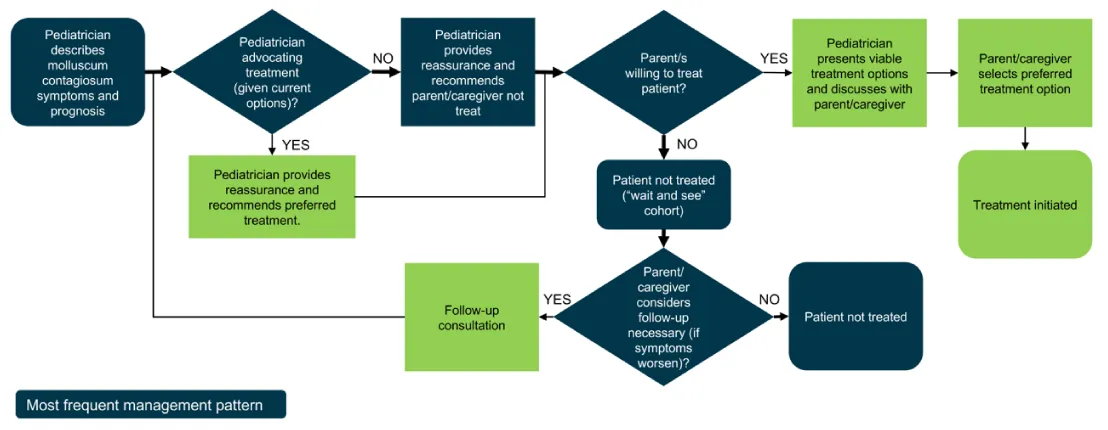
If the pediatrician treated, over half used prescription topicals (56%, n = 14) followed by cryotherapy (52%, n = 13), over-the-counter topicals 36% (n = 9) and about one-third (32%, n = 8) curettage/physical removal. Homeopathy or natural medicine was used by 16% (n = 4). The type of MC therapy the pediatrician chose was influenced by case-specific factors including age, lesion location and count, and level of urgency for resolution; pediatricians generally present all options and leave the final decision to the patient/parent (Figure 2).
When queried about the need for new MC treatment options, most pediatricians interviewed agreed there is a therapeutic gap as current regimens have limited efficacy, are not FDA-approved, and are associated with bothersome side effects. A new MC therapy that could ease parental anxiety and reduce the duration of MC was desirable. Regarding the decision to prescribe, the three most important attributes of a potential new MC therapy were safety, efficacy, and side effects. The least important attribute was the onset of action.
Respondents were not aware of MC management guidelines but said they would find guidelines useful, particularly if issued by the American Academy of Pediatrics or the American Academy of Dermatology.
Discussion
MC is a contagious skin infection primarily affecting children and adolescents. Pediatricians are often the first healthcare providers to diagnose molluscum; however, most take a watch-and-wait approach to treatment. Although MC lesions may clear in a few months, some episodes may last several months to years, with the infected child remaining contagious while active lesions are present.
To date, there are no professional practice guidelines endorsed for the management of MC, and only two recently approved therapies, Y-Canth™ (0.7% cantharidin, Verrica, Westchester, PA), a drug-device combination of a blistering agent requiring application in a healthcare provider’s office and post-application wash off [5] and Zelsuvmi ™ (berdazimer topical gel, 10.3%, LNHC, Durham, NC), a first-in-class topical nitric oxide-releasing prescription medication approved in January 2024, that is applied by the patient or caregiver once a day and does not require post-application removal [6,7]. Prior to these approvals, there had been an unmet need for a well-studied, safe, effective, and convenient MC treatment. This study sought to capture pediatric practices in the treatment of MC, to understand key drivers in therapy initiation, and to identify therapeutic gaps.
Consistent with Red Book guidance [2], this exploratory in-depth interview revealed most pediatricians take a wait-and-see approach for patients with MC. However, clinicians are more willing to treat if desired by a parent/caregiver. The most commonly cited treatments were topical prescription therapies followed by cryotherapy. Treatment selection varied based on patient attributes, including age, anatomical location of lesions, and number of lesions. Referrals to other specialists were limited but more likely if the patient required more extensive therapy, suggesting a multidisciplinary approach to challenging MC cases.
Parental anxiety was revealed to be the primary reason MC therapy was initiated. This result was consistent with Olsen and colleagues [8] finding that 82% of parents (23/30) were moderately or greatly concerned about their child’s molluscum, and physician-driven reassurance about the child’s MC disease course was paramount to alleviating parental concern.
In clinical challenges such as these, professional practice guidelines often prove helpful in standardizing care and avoiding unnecessary off-label use of ineffective therapies. MC management guidelines exist in the European Union but not in the United States [9]. Development of US MC management guidelines, spearheaded by the American Academy of Pediatrics and/or the American Academy of Dermatology, could help clinicians better communicate and reassure parents about effective treatment strategies aimed at reducing the risk of re-infection or viral spread and provide much-needed guidance regarding evidence-based standards of care, particularly in cases of widespread, persistent, or bothersome MC.
Limitations
A small sample of pediatricians was queried, and the survey questionnaire was not validated. Nevertheless, the results provide important insights into the usual care of patients with MC in pediatric practices. This interview (conducted in 2022) highlighted the lack of FDA-approved MC treatment options at the time, the desire for safe, effective, and convenient treatments, and the strong influence of parental engagement in MC treatment selection. A larger study using a validated questionnaire may be useful to identify specific educational gaps or strategies that could be used by clinicians to foster adherence to MC mitigation practices among parents/caregivers and those infected with molluscum. No pediatric dermatologists at academic medical centers were surveyed to discern if this specialty manages MC differently from local pediatricians.
Conclusion
The interview data herein shed light on practice patterns among pediatricians and confirm that most do not treat MC infection but rather wait for it to resolve. However, when prompted by parental concern, treatment, albeit off-label, is sometimes initiated. None of the treatments used by pediatricians at the time of the survey were FDA-approved and there appeared to be no specific criteria regarding which cases of MC merited treatment. MC management practice patterns are expected to change with the recent approval of two FDA-approved therapies. The development of expert-driven, evidence-based MC management guidelines is encouraged to standardize the treatment of MC in children.
Conflicts of interest
Drs. Cartwright and Maeda-Chubachi and Ms. Enloe are employees of Pelthos (previously Novan, Inc.). Dr. Stripling is a clinical investigator and advisor for Pelthos, and a clinical investigator for Arcutis Biotherapeutics. Dr. Hebert has received research grants paid to the McGovern School of Medicine from AbbVie, Arcutis, Pelthos, and Pfizer; has received honoraria from Pfizer, Arcutis, Incyte, Pelthos, Ortho Dermatologics, Amyrt, Galderma, Almirall; and is a member of Data Safety Monitoring Boards for GSK, Ortho Dermatologics, and Sanofi Regeneron.
Funding: The pediatrician interviews and manuscript development were funded by Pelthos (previously Novan) Durham, NC, USA. Novan/Pelthos contracted Syneos Health Consulting to design and conduct interviews and collate and analyze the data.
Author contributions: Drs. Cartwright and Maeda-Chubachi conceptualized and designed the study. Dr. Cartwright drafted the initial manuscript. Drs. Maeda-Chubachi, Stripling, and Hebert, and Ms. Enloe critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013 Oct;13(10):877-88. doi: 10.1016/S1473-3099(13)70109-9. Epub 2013 Aug 21. PMID: 23972567.
- Committee on Infectious Diseases. American Academy of Pediatrics. Red Book: 2021-2024. Report of the Committee on Infectious Diseases. 32nd ed. American Academy of Pediatrics; 2021.
- American Academy of Dermatology. Molluscum contagiosum: an overview. Accessed December 16, 2022. https://www.aad.org/public/diseases/a-z/molluscum-contagiosum-overview
- van der Wouden JC, van der Sande R, Kruithof EJ, Sollie A, van Suijlekom-Smit LW, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2017 May 17;5(5):CD004767. doi: 10.1002/14651858.CD004767.pub4. PMID: 28513067; PMCID: PMC6481355.
- Verrica Pharmaceuticals Inc. YCANTH™ (cantharidin) topical solution. 2023. https://ycanth.com/wp-content/uploads/2023/07/USPI_FPI-0003_YCANTH.pdf. Accessed 8 September 2023
- LNHC Inc. ZELSUVMI™ (berdazimer) topical gel [prescribing information]. January 2024.
- US Food and Drug Administration. Novel drug approvals for 2024. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2024
- Olsen JR, Piguet V, Gallacher J, Francis NA. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016 Jan;66(642):e53-8. doi: 10.3399/bjgp15X688093. Epub 2015 Dec 6. PMID: 26639950; PMCID: PMC4684036.
- Edwards S, Boffa MJ, Janier M, Calzavara-Pinton P, Rovati C, Salavastru CM, Rongioletti F, Wollenberg A, Butacu AI, Skerlev M, Tiplica GS. 2020 European guideline on the management of genital molluscum contagiosum. J Eur Acad Dermatol Venereol. 2021 Jan;35(1):17-26. doi: 10.1111/jdv.16856. Epub 2020 Sep 2. PMID: 32881110.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
