Open Journal of Pediatrics and Child Health
The clinical role of probiotic and prebiotic supplementations in preterm infants
Giulio Perrotta*
Cite this as
Perrotta G (2023) The clinical role of probiotic and prebiotic supplementations in preterm infants. Open J Pediatr Child Health 8(1): 007-014. DOI: 10.17352/ojpch.000046Copyright
© 2023 Perrotta G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: For over two decades we have been trying to study and demonstrate the role of the gut microbiota in the onset of cardiovascular, autoimmune, infectious and neurobiological diseases and more generally the clinical efficacy.
Aims: To study the clinical efficacy of the integrative use of prebiotics and probiotics in the prenatal population.
Materials and methods: All clinical trials and randomized controlled trials were selected through January 6, 2023, for a useful total of 32 studies and a cohort of more than 37,000 infants, of which just under half are term infants in the control groups.
Results: In the neonatal literature, studies on the clinical use of prebiotics and probiotics focus on specific topics of investigation, starting from the intestinal microbial composition and then extending the object of analysis to the effects of antibiotics on the microbiota, to the biochemical integration of these products, the use of breast milk or artificial or donor milk, the alleged claim to intervene on pathological processes arising from opportunistic infections of the respiratory tract, and also in relation to autoimmune, gastrointestinal and dermatological pathologies, up to food intolerances.
Conclusions: Significant evidence emerges in the literature that supports the therapeutic use for clinical purposes of prebiotics and probiotics even in neonatology; however, most of the published studies have structural and functional criticalities that often invalidate the research design and therefore the outcome obtained and published, risking to affect negatively the significance eventually detected. Further studies are needed that can confirm and expand scientific knowledge in this particular area.
Background and aims
For over twenty years we have been trying to study and demonstrate the role of the intestinal microbiota in the onset of cardiovascular, autoimmune, infectious and neurobiological diseases, and more generally the clinical efficacy of the integrative use of prebiotics and probiotics in human nutrition in order to reduce the onset of symptoms or prevent the pathological course. Therefore, there is a clinical need to answer these questions with greater certainty, in order to clarify the exact dynamics of the use of prebiotics and probiotics and their therapeutic interactions with allegedly related disorders, particularly in the neonatal setting on preterm infants (born before 36 weeks and with a birth weight less than 1500 grams) where the hypothesis of use for preventive and curative purposes appears to be not only a speculative suggestion.
Materials and methods
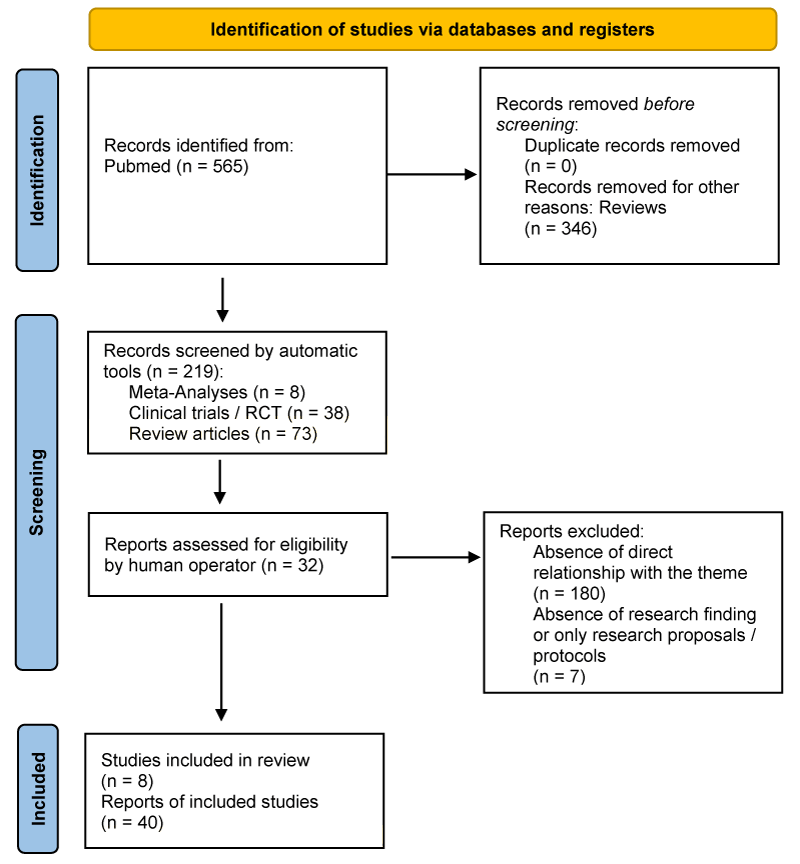
We searched on Pubmed until January 30, 2023, clinical trials and randomized controlled trials using in combination the keywords “gut microbiota”, “preterm infants”, “prebiotic” and “probiotic”, selecting 219 useful results. To these were also added 8 reviews related to the last two years, in order to have a greater and complete overview of the topic, ultimately selecting a total of 40 research and studies, for a total population of over 37,000 infants, of which slightly less than half are represented by term infants in the control groups. Simple reviews, opinion contributions, or publications in popular volumes were excluded because they were not relevant or redundant for the purposes of this work, and 7 types of research of the final total because they did not present results or statistical samples but only protocol and research proposals [1-3], did not specifically address the relationship between the gut microbiota and preterm infants [4], the data were contradictory, unreliable, or otherwise, the research design had functional shortcomings [5], or the study sample was not directly preterm infants [6,7].
The search not was limited to English-language papers. No limit on the year of publication was set. We limited the search by applying the age filter “newborns” and used the following search terms and rationale: “preterm infants microbiota,” “gastrointestinal microbiome AND necrotizing enterocolitis or NEC”, “breastfeeding and enteral nutrition AND necrotizing enterocolitis OR NEC”, “microbiota AND growth retardation”, “gut microbiota AND weight gain”, “gut microbiota AND growth”, “gut microbiota AND extrauterine growth restriction”, “microbiota of preterm infants AND late-onset sepsis OR LOS”, “microbiota OR microbiome OR bacteria OR antibiotics OR gut AND lung OR airway OR BPD OR bronchopulmonary dysplasia” and “gut-lung axis” (Figure 1).
Results
In the neonatal literature, studies on the intestinal microbiota focus on specific topics of investigation, starting from its composition and then extending the object of analysis to the effects of antibiotics on the microbiota, to the biochemical integration of prebiotics, probiotics and postbiotics, the use of breast milk or artificial or donor milk, up to the alleged claim to intervene on pathological processes arising from opportunistic infections of the respiratory tract, or even in relation to autoimmune, allergological, endocrinological, gastrointestinal and dermatological pathologies.
In a preterm infant, during the first 60 days of life, there is an intestinal microbial composition in which first Staphylococcus, then Enterococcus (which are able to delay the subsequent stages of development of the microbiota) and Enterobacter predominate, and then stabilize with Bifidobacterium, as happens in infants born at term, fed with breast milk and free of clinical complications [8]. The predominance of Bifidobacterium is correlated in several studies to the good health of the infant [9] or at least to the improvement of the clinical picture and an increase in weight [10], mainly as a result of the decrease of Enterobacter and Clostridium colonies [11]. The abundance of Candida-like Saccharomycetes is often present in the microbiota of the preterm infant and therefore could be a contributing cause of the condition [12]. What consistently emerges is that the intestinal microbiota tends to change, favoring dysbiotic processes in the hypothesis of intrapartum exposure, antibiotic use, premature birth, duration, and type of breastfeeding [9]. Research has then attentively addressed the need to improve the identification of the viable microbiota in order to increase the accuracy of clinical inferences made regarding the impact of the preterm gut microbiota on health and disease; this study validates the use of Propidium Monoazide (PMA), a DNA chelating agent that is excluded from an intact bacterial membrane, to reduce the bias associated with 16S rRNA gene analysis of clinical stool samples. Indeed, the meta-analysis showed a significant reduction in bacterial diversity in 68.8% of PMA-treated samples, as well as a significantly reduced overall abundance of rare taxa. Importantly, the overall abundances of genera associated with protection and propensity for necrotizing enterocolitis and sepsis such as Bifidobacterium, Clostridium and Staphylococcus sp. were significantly different after PMA treatment [10].
But it is the lack of breast milk intake, in particular, that appears to be related to several circumstances that promote gut dysbiosis: a) preterm infants who receive breast milk develop a gut microbiota very similar to that of infants born at term who are equally breastfed [8], with a predominance of Bifidobacterium and Bacteroides and a consequent 60% reduction in food intolerances, significant weight gain and greater gut microbial biodiversity, even compared with donor milk that has a greater presence of Staphylococcus [13,14]; b) in both preterm and term infants, evidence emerges of a deficiency of metabolites present in breast milk, such as fucose and β-hydroxybutyrate, which predisposes to the metabolic syndrome and food allergies [15,16]; breast milk, when administered to the adult, precisely because it is rich in oligosaccharides (HMOs) capable of significantly shaping the intestinal microbiota by selectively stimulating the growth of specific bacteria (with immune and gastrointestinal benefits), produces substantial increases in the abundance of Actinobacteria and Bifidobacterium, and a reduction in Firmicutes and Proteobacteria [17], and therefore also in the preterm infant has the same positive efficacy [18].
Other studies on the intestinal microbial composition have been done starting from dietary supplementation of different mixtures (other than breast milk); compared to standard nutritional therapy: a) addition of a High Polyunsaturated Fatty Acid (HF-PUFA) blend of fish oil and safflower oil resulted in increased bacterial biodiversity and lower abundance of Streptococcus, Clostridium, and many pathogenic genera within the Enterobacteriaceae family, positively correlating with normal metabolic processes of amino acids, carbohydrates, fatty acids and secondary bile acid synthesis [19]; b) the integration of bovine lactoferrin, a glycoprotein with recognized antibacterial, antiviral, anti-inflammatory and protective faculties of the gastrointestinal system [20], to the diet of the preterm infant, does not alter the intestinal flora, neither on its composition nor on its function, thus excluding that it can generate dysbiosis as it happens with the administration of antibiotics [16], since as for the term newborn it is able to increase Citrobacter and reduce Enterobacterium (and in particular Klebsiella) and Firmicutes (in particular Staphylococcus), responsible for many neonatal infections [21]; c) dietary supplementation of bee honey for medical purposes produces a modification of the intestinal microbiota by increasing Bifidobacterium and Lactobacillus, resulting in increased body weight, improved clinical picture and growth values, although it does not impact on the inflammatory profile detectable by the presence of CD4 and CD8 cytokines [22]; d) the mixture of fermented milk and supplemented with probiotic strains of Bifidobacterium breve C50 and Streptococcus thermophilus 065 does not impact on bifidus but significantly decreases the values of fecal calprotectic, actually intervening on intestinal inflammatory states [23]; e) Galacto-Oligosaccharide/Polydextrose (GOS/PDX) mixture positively impacted on respiratory infections and atopic dermatitis [24], as Bifidobacterium have a protective role in respiratory infections while Clostridium in atopic dermatitis [25]; f) breast milk is the best option to avoid or reduce the risk of necrotizing enterocolitis but in its absence bovine colostrum is the best option even compared to artificial and human donor milk, as it stimulates intestinal immunity and digestive functions more, although causing transient increased thyroxine [26].
Other profiles studied in research concern the use of antibiotics in premature infants and their interaction with the intestinal microbiota. It has been shown that a single course of antibiotics has a limited effect on the microbial composition [27] but if prolonged or repeated negatively impacts by promoting the increase of pathogenic bacterial strains (as in the case of Proteobacter type E. coli and Helicobacter pylori) and therefore dysbiotic processes that increase the onset of gastrointestinal, infectious and immune complications, in some cases even fatal as necrotizing enterocolitis and sepsis [28,29].
In fact, some probiotics used in neonatology would appear to positively affect specific morbid conditions, just as necrotizing enterocolitis and sepsis. Research on a sample of more than 10,000 preterm infants, who participated in randomized controlled trials of probiotics worldwide, suggests that probiotics in general could reduce the rates of necrotizing enterocolitis, sepsis and mortality, without however indicating specific techniques about the related bacterial strain to be used, the correct dosage and time of administration. Questions remain about the guarantee of the product used, the certification of the strain used for the absence of antibiotic-resistant genes, and the possible risk of probiotic sepsis [30]. Another study analyzed data from 15,712 preterm infants, and compared with a placebo, a combination of 1 or more Lactobacillus species (spp) and one or more Bifidobacterium spp was the only intervention with moderate or high-quality evidence of reducing all-cause mortality. Interventions with moderate or high-quality evidence of efficacy compared with placebo included combinations of: a) one or more Lactobacillus spp and one or more Bifidobacterium spp, Bifidobacterium animalis subspecies lactis, Lactobacillus reuteri or Lactobacillus rhamnosus is able to significantly reduce severe necrotizing enterocolitis [31], whereas administration of Bifidobacterium breve has been shown to be futile [29]; b) one or more Lactobacillus spp and one or more Bifidobacterium spp and Saccharomyces boulardii, which can reduce the number of days to reach full nutrition [31]; c) the monospecific product B animalis subsp lactis or L reuteri, which can significantly reduce the duration of hospitalization [31]; d) combined probiotic supplementation of Bifidobacterium longum subsp. infantis BB-02, Streptococcus thermophilus TH-4 and Bifidobacterium animalis subsp. lactis BB-12 reduces the presence of Enterococcus favoring a 54% case-controlled reduction of necrotizing enterocolitis [32]; e) combined probiotic supplementation of Bifidobacterium is able to alleviate dysbiosis (both in single and multiple administration) [33] and reduce the consequences of late sepsis [34]; the mixture of prebiotics and probiotics based on galacto-oligosaccharides and polydextrose 1:1 and Lactobacillus rhamnosus GG can alleviate symptoms of agitation and crying in the preterm infant resulting from gastrointestinal disorders such as colic [35] and also positively impact respiratory rhinovirus infections [25].
However, there are studies that have completely opposite results, albeit with extremely small and non-significant population samples, which support the little or total uselessness in the use of prebiotics and probiotics, both in terms of incidence of the pathogenic microbial amount and in terms of weight gain or length of hospital stay [36,37]; studies that are then disproved or otherwise challenged by meta-analysis with large and representative samples: a recent study, in fact, which included 12,320 participants, supports the importance of the association of Bifidobacterium and Lactobacillus in therapeutic use, to reinforce the beneficial effects and decrease mortality rates [38]. Observational studies then demonstrating reduced rates of infection, necrotizing enterocolitis and mortality in preterm infants fed breast milk, compared to formula, prompted attempts to achieve similar effects with the right choice of food and food additives; the use then of prebiotic oligosaccharides found reduced infection but not mortality, as enteral L-glutamine reduced infection rates and enteral L-arginine reduced the morbid condition [39,40] (Table 1).
Discussion and limitations
Beyond the easy enthusiasm, most of the published studies have structural and functional criticalities that often invalidate the research design and therefore the outcome obtained and published, risking to affect negatively the significance eventually detected. The main criticalities refer to the following circumstances: a) the population sample selected is too limited and therefore not representative, almost always below 100 units; b) the variables that can trigger the dysbiotic process are multiple and not all known, as well as the etiological causes underlying the onset or aggravation of a disease and therefore correlate the use of probiotic or a specific mixture is not always possible without considering the risk of bias or oversimplification of a given clinical problem; c) in few studies, the authors report anthropometric data of newborns, such as birth weight, gestational age and size of body districts, in full; d) in almost all studies there is a lack of publication of technical data about the related bacterial strain to be used, the correct dosage and time of administration, as well as safety data regarding the guarantee of the product used, the certification of the microbial strain about the absence of antibiotic-resistant genes and the possible risk of probiotic sepsis; e) the economic interests on the production of a specific integrative product, patented or to be patented, which in the best cases can favor the conditioning need of the investigators to confirm the scientific validity. This representative approach is more reinforced in the clinical setting, both for neonatal and pediatric as well as adult, precisely because of the increasing difficulty concerning the ability to objectively analyze the multifactorial landscape according to a holistic key.
Conclusion
Significant evidence emerges in the literature, that supports the therapeutic use for clinical purposes of prebiotics and probiotics even in neonatology, of specific bacterial strains of Lactobacillus, Bifidobacterium, and Saccharomyces boulardii to reduce the risk of NEC, sepsis and systemic infections. In addition, the use of mixtures of High Polyunsaturated Fatty Acids (HF-PUFA) from fish oil and safflower oil, galacto-oligosaccharides and polydextrose 1 appear useful: 1, bovine lactoferrin, bee honey, fermented milk supplemented with probiotic strains, Galacto-Oligosaccharides/Polydextrose (GOS/PDX), and bovine colostrum to strengthen and support the immune system, as well as the use of Propidium Monoazide (PMA) as a DNA chelating agent to reduce the bias associated with 16S rRNA gene analysis of clinical stool samples. These data are consistent with results obtained with adult patients, confirming the importance of using prebiotics and probiotics, for clinical purposes, from pregnancy and birth.
It emerges, therefore, the need, in such a varied and contradictory landscape, to design a research project that takes into account first of all a significant and representative sample of the population, but above all that does not underestimate the critical issues mentioned above, in order to address with scientific method the proper and functional use of prebiotics and probiotics in neonatal and pediatric in general; therefore, further studies are needed that can confirm and expand scientific knowledge in this particular field.
- Barthow C, Wickens K, Stanley T, Mitchell EA, Maude R, Abels P, Purdie G, Murphy R, Stone P, Kang J, Hood F, Rowden J, Barnes P, Fitzharris P, Craig J, Slykerman RF, Crane J. The Probiotics in Pregnancy Study (PiP Study): rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth. 2016 Jun 3;16(1):133. doi: 10.1186/s12884-016-0923-y. PMID: 27255079; PMCID: PMC4891898.
- Cooijmans KHM, Beijers R, Rovers AC, de Weerth C. Effectiveness of skin-to-skin contact versus care-as-usual in mothers and their full-term infants: study protocol for a parallel-group randomized controlled trial. BMC Pediatr. 2017 Jul 6;17(1):154. doi: 10.1186/s12887-017-0906-9. PMID: 28683833; PMCID: PMC5501342.
- Yu J, Wells J, Wei Z, Fewtrell M. Effects of relaxation therapy on maternal psychological state, infant growth and gut microbiome: protocol for a randomised controlled trial investigating mother-infant signalling during lactation following late preterm and early term delivery. Int Breastfeed J. 2019 Dec 16;14:50. doi: 10.1186/s13006-019-0246-5. PMID: 31889973; PMCID: PMC6916017.
- Fischer N, Darmstadt GL, Shahunja KM, Crowther JM, Kendall L, Gibson RA, Ahmed T, Relman DA. Topical emollient therapy with sunflower seed oil alters the skin microbiota of young children with severe acute malnutrition in Bangladesh: A randomised, controlled study. J Glob Health. 2021 Jul 17;11:04047. doi: 10.7189/jogh.11.04047. PMID: 34386216; PMCID: PMC8325932.
- Chong CYL, Vatanen T, Alexander T, Bloomfield FH, O'Sullivan JM. Factors Associated With the Microbiome in Moderate-Late Preterm Babies: A Cohort Study From the DIAMOND Randomized Controlled Trial. Front Cell Infect Microbiol. 2021 Mar 1;11:595323. doi: 10.3389/fcimb.2021.595323. PMID: 33732655; PMCID: PMC7958882.
- Di Pierro F, Parolari A, Brundu B, Nigro R. Positive clinical outcomes derived from using a proprietary mixture of selected strains during pregnancy. Acta Biomed. 2016 Jan 16;87(3):259-265. PMID: 28112691.
- Deianova N, El Manouni El Hassani S, Niemarkt HJ, Cossey V, van Kaam AH, Jenken F, van Weissenbruch MM, Doedes EM, Baelde K, Menezes R, Benninga MA, de Jonge WJ, de Boer NK, de Meij TG. Fecal Volatile Organic Compound Profiles are Not Influenced by Gestational Age and Mode of Delivery: A Longitudinal Multicenter Cohort Study. Biosensors (Basel). 2020 May 11;10(5):50. doi: 10.3390/bios10050050. PMID: 32403393; PMCID: PMC7277672.
- Korpela K, Blakstad EW, Moltu SJ, Strømmen K, Nakstad B, Rønnestad AE, Brække K, Iversen PO, Drevon CA, de Vos W. Intestinal microbiota development and gestational age in preterm neonates. Sci Rep. 2018 Feb 6;8(1):2453. doi: 10.1038/s41598-018-20827-x. PMID: 29410448; PMCID: PMC5802739.
- Forsgren M, Isolauri E, Salminen S, Rautava S. Late preterm birth has direct and indirect effects on infant gut microbiota development during the first six months of life. Acta Paediatr. 2017 Jul;106(7):1103-1109. doi: 10.1111/apa.13837. Epub 2017 Apr 24. PMID: 28316118; PMCID: PMC5763336.
- Young GR, Smith DL, Embleton ND, Berrington JE, Schwalbe EC, Cummings SP, van der Gast CJ, Lanyon C. Reducing Viability Bias in Analysis of Gut Microbiota in Preterm Infants at Risk of NEC and Sepsis. Front Cell Infect Microbiol. 2017 Jun 6;7:237. doi: 10.3389/fcimb.2017.00237. PMID: 28634574; PMCID: PMC5459914.
- Blakstad EW, Korpela K, Lee S, Nakstad B, Moltu SJ, Strømmen K, Rønnestad AE, Brække K, Iversen PO, de Vos WM, Drevon CA. Enhanced nutrient supply and intestinal microbiota development in very low birth weight infants. Pediatr Res. 2019 Sep;86(3):323-332. doi: 10.1038/s41390-019-0412-x. Epub 2019 May 14. PMID: 31086354.
- Mohan R, Koebnick C, Schildt J, Schmidt S, Mueller M, Possner M, Radke M, Blaut M. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: a double-blind, placebo-controlled, randomized study. J Clin Microbiol. 2006 Nov;44(11):4025-31. doi: 10.1128/JCM.00767-06. Epub 2006 Sep 13. PMID: 16971641; PMCID: PMC1698302.
- Willis KA, Purvis JH, Myers ED, Aziz MM, Karabayir I, Gomes CK, Peters BM, Akbilgic O, Talati AJ, Pierre JF. Fungi form interkingdom microbial communities in the primordial human gut that develop with gestational age. FASEB J. 2019 Nov;33(11):12825-12837. doi: 10.1096/fj.201901436RR. Epub 2019 Aug 31. PMID: 31480903; PMCID: PMC6902694.
- Ford SL, Lohmann P, Preidis GA, Gordon PS, O'Donnell A, Hagan J, Venkatachalam A, Balderas M, Luna RA, Hair AB. Improved feeding tolerance and growth are linked to increased gut microbial community diversity in very-low-birth-weight infants fed mother's own milk compared with donor breast milk. Am J Clin Nutr. 2019 Apr 1;109(4):1088-1097. doi: 10.1093/ajcn/nqz006. PMID: 30982856; PMCID: PMC6462428.
- Yap PSX, Chong CW, Ahmad Kamar A, Yap IKS, Choo YM, Lai NM, Teh CSJ. Neonatal intensive care unit (NICU) exposures exert a sustained influence on the progression of gut microbiota and metabolome in the first year of life. Sci Rep. 2021 Jan 14;11(1):1353. doi: 10.1038/s41598-020-80278-1. Erratum in: Sci Rep. 2021 Apr 21;11(1):9085. PMID: 33446779; PMCID: PMC7809424.
- Qiao LX, Zhu WY, Zhang HY, Wang H. Effect of early administration of probiotics on gut microflora and feeding in pre-term infants: a randomized controlled trial. J Matern Fetal Neonatal Med. 2017 Jan;30(1):13-16. doi: 10.3109/14767058.2016.1163674. Epub 2016 Mar 29. PMID: 26956782.
- Elison E, Vigsnaes LK, Rindom Krogsgaard L, Rasmussen J, Sørensen N, McConnell B, Hennet T, Sommer MO, Bytzer P. Oral supplementation of healthy adults with 2'-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Br J Nutr. 2016 Oct;116(8):1356-1368. doi: 10.1017/S0007114516003354. Epub 2016 Oct 10. PMID: 27719686; PMCID: PMC5082288.
- Underwood MA, Gaerlan S, De Leoz ML, Dimapasoc L, Kalanetra KM, Lemay DG, German JB, Mills DA, Lebrilla CB. Human milk oligosaccharides in premature infants: absorption, excretion, and influence on the intestinal microbiota. Pediatr Res. 2015 Dec;78(6):670-7. doi: 10.1038/pr.2015.162. Epub 2015 Aug 31. PMID: 26322410; PMCID: PMC4689671.
- Younge N, Yang Q, Seed PC. Enteral High Fat-Polyunsaturated Fatty Acid Blend Alters the Pathogen Composition of the Intestinal Microbiome in Premature Infants with an Enterostomy. J Pediatr. 2017 Feb;181:93-101.e6. doi: 10.1016/j.jpeds.2016.10.053. Epub 2016 Nov 15. PMID: 27856001; PMCID: PMC5274578.
- Campione E, Cosio T, Rosa L, Lanna C, Di Girolamo S, Gaziano R, Valenti P, Bianchi L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int J Mol Sci. 2020 Jul 11;21(14):4903. doi: 10.3390/ijms21144903. PMID: 32664543; PMCID: PMC7402319.
- Sherman MP, Sherman J, Arcinue R, Niklas V. Randomized Control Trial of Human Recombinant Lactoferrin: A Substudy Reveals Effects on the Fecal Microbiome of Very Low Birth Weight Infants. J Pediatr. 2016 Jun;173 Suppl:S37-42. doi: 10.1016/j.jpeds.2016.02.074. PMID: 27234409.
- Aly H, Said RN, Wali IE, Elwakkad A, Soliman Y, Awad AR, Shawky MA, Alam MSA, Mohamed MA. Medically Graded Honey Supplementation Formula to Preterm Infants as a Prebiotic: A Randomized Controlled Trial. J Pediatr Gastroenterol Nutr. 2017 Jun;64(6):966-970. doi: 10.1097/MPG.0000000000001597. PMID: 28379925.
- Chrzanowska-Liszewska D, Seliga-Siwecka J, Kornacka MK. The effect of Lactobacillus rhamnosus GG supplemented enteral feeding on the microbiotic flora of preterm infants-double blinded randomized control trial. Early Hum Dev. 2012 Jan;88(1):57-60. doi: 10.1016/j.earlhumdev.2011.07.002. Epub 2011 Nov 4. PMID: 22055271.
- Luoto R, Ruuskanen O, Waris M, Kalliomäki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014 Feb;133(2):405-13. doi: 10.1016/j.jaci.2013.08.020. Epub 2013 Oct 13. PMID: 24131826; PMCID: PMC7112326.
- Ranucci G, Buccigrossi V, Borgia E, Piacentini D, Visentin F, Cantarutti L, Baiardi P, Felisi M, Spagnuolo MI, Zanconato S, Baraldi E, Giaquinto C, Guarino A. Galacto-Oligosaccharide/Polidextrose Enriched Formula Protects against Respiratory Infections in Infants at High Risk of Atopy: A Randomized Clinical Trial. Nutrients. 2018 Mar 1;10(3):286. doi: 10.3390/nu10030286. PMID: 29494489; PMCID: PMC5872704.
- Juhl SM. Necrotizing enterocolitis - classification and two initial steps towards prevention. Dan Med J. 2017 Jun;64(6):B5362. PMID: 28566122.
- Kim CS, Grady N, Derrick M, Yu Y, Oliphant K, Lu J, Claud EC. Effect of Antibiotic Use Within First 48 Hours of Life on the Preterm Infant Microbiome: A Randomized Clinical Trial. JAMA Pediatr. 2021 Mar 1;175(3):303-305. doi: 10.1001/jamapediatrics.2020.4916. PMID: 33196773; PMCID: PMC7670395.
- Russell JT, Lauren Ruoss J, de la Cruz D, Li N, Bazacliu C, Patton L, McKinley KL, Garrett TJ, Polin RA, Triplett EW, Neu J. Antibiotics and the developing intestinal microbiome, metabolome and inflammatory environment in a randomized trial of preterm infants. Sci Rep. 2021 Jan 21;11(1):1943. doi: 10.1038/s41598-021-80982-6. PMID: 33479274; PMCID: PMC7820285.
- Millar M, Seale J, Greenland M, Hardy P, Juszczak E, Wilks M, Panton N, Costeloe K, Wade WG. The Microbiome of Infants Recruited to a Randomised Placebo-controlled Probiotic Trial (PiPS Trial). EBioMedicine. 2017 Jun;20:255-262. doi: 10.1016/j.ebiom.2017.05.019. Epub 2017 May 17. PMID: 28571671; PMCID: PMC5478240.
- van den Akker CHP, van Goudoever JB, Shamir R, Domellöf M, Embleton ND, Hojsak I, Lapillonne A, Mihatsch WA, Berni Canani R, Bronsky J, Campoy C, Fewtrell MS, Fidler Mis N, Guarino A, Hulst JM, Indrio F, Kolaček S, Orel R, Vandenplas Y, Weizman Z, Szajewska H. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2020 May;70(5):664-680. doi: 10.1097/MPG.0000000000002655. PMID: 32332478.
- Morgan RL, Preidis GA, Kashyap PC, Weizman AV, Sadeghirad B; McMaster Probiotic, Prebiotic, and Synbiotic Work Group. Probiotics Reduce Mortality and Morbidity in Preterm, Low-Birth-Weight Infants: A Systematic Review and Network Meta-analysis of Randomized Trials. Gastroenterology. 2020 Aug;159(2):467-480. doi: 10.1053/j.gastro.2020.05.096. Epub 2020 Jun 24. PMID: 32592699; PMCID: PMC8014956.
- Plummer EL, Bulach DM, Murray GL, Jacobs SE, Tabrizi SN, Garland SM; ProPrems Study Group. Gut microbiota of preterm infants supplemented with probiotics: sub-study of the ProPrems trial. BMC Microbiol. 2018 Nov 13;18(1):184. doi: 10.1186/s12866-018-1326-1. PMID: 30424728; PMCID: PMC6234596.
- Athalye-Jape G, Esvaran M, Patole S, Simmer K, Nathan E, Doherty D, Keil A, Rao S, Chen L, Chandrasekaran L, Kok C, Schuster S, Conway P. Effect of single versus multistrain probiotic in extremely preterm infants: a randomised trial. BMJ Open Gastroenterol. 2022 Feb;9(1):e000811. doi: 10.1136/bmjgast-2021-000811. PMID: 35185013; PMCID: PMC8860036.
- Berkhout DJC, Niemarkt HJ, Buijck M, van Weissenbruch MM, Brinkman P, Benninga MA, van Kaam AH, Kramer BW, Andriessen P, de Boer NKH, de Meij TGJ. Detection of Sepsis in Preterm Infants by Fecal Volatile Organic Compounds Analysis: A Proof of Principle Study. J Pediatr Gastroenterol Nutr. 2017 Sep;65(3):e47-e52. doi: 10.1097/MPG.0000000000001471. PMID: 27846067.
- Pärtty A, Luoto R, Kalliomäki M, Salminen S, Isolauri E. Effects of early prebiotic and probiotic supplementation on development of gut microbiota and fussing and crying in preterm infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2013 Nov;163(5):1272-7.e1-2. doi: 10.1016/j.jpeds.2013.05.035. Epub 2013 Jul 31. PMID: 23915796.
- Campeotto F, Suau A, Kapel N, Magne F, Viallon V, Ferraris L, Waligora-Dupriet AJ, Soulaines P, Leroux B, Kalach N, Dupont C, Butel MJ. A fermented formula in pre-term infants: clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br J Nutr. 2011 Jun 28;105(12):1843-51. doi: 10.1017/S0007114510005702. Epub 2011 Mar 22. PMID: 21426607.
- Zeber-Lubecka N, Kulecka M, Ambrozkiewicz F, Paziewska A, Lechowicz M, Konopka E, Majewska U, Borszewska-Kornacka M, Mikula M, Cukrowska B, Ostrowski J. Effect of Saccharomyces boulardii and Mode of Delivery on the Early Development of the Gut Microbial Community in Preterm Infants. PLoS One. 2016 Feb 26;11(2):e0150306. doi: 10.1371/journal.pone.0150306. PMID: 26918330; PMCID: PMC4769247.
- Chi C, Li C, Buys N, Wang W, Yin C, Sun J. Effects of Probiotics in Preterm Infants: A Network Meta-analysis. Pediatrics. 2021 Jan;147(1):e20200706. doi: 10.1542/peds.2020-0706. Epub 2020 Dec 15. PMID: 33323491.
- Bührer C, Fischer HS, Wellmann S. Nutritional interventions to reduce rates of infection, necrotizing enterocolitis and mortality in very preterm infants. Pediatr Res. 2020 Jan;87(2):371-377. doi: 10.1038/s41390-019-0630-2. Epub 2019 Oct 23. PMID: 31645057.
- Perrotta G. The intestinal microbiota: towards a multifactorial integrative model. Eubiosis and dysbiosis in morbid physical and psychological conditions. Arch Clin Gastroenterol. 2021; 7(2): 024-035.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
